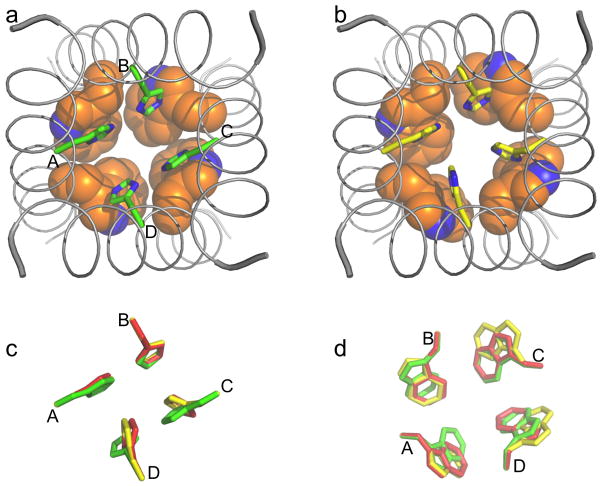
Fig. 4.
Models of the His37-Trp41 quartet under neutral and acidic pH. (a) D2 (+2 charge), representing the histidine-locked state at neutral pH. (b) D4 (+3 charge), representing the conducting state at acidic pH. His37 sidechains are in stick mode and Trp41 sidechains are in space-filling mode; the backbone structure of the TM domain (from 3LBW) is also shown. (c) and (d): Superposition of the His37 and Trp41 sidechains in D2 (green), D3 (+3 charge; red), and D4 (yellow). These conformations mainly differ in His37 χ2 angles of chains C and D and Trp41 χ1 angles of chains A, C, and D.