Figure 1.
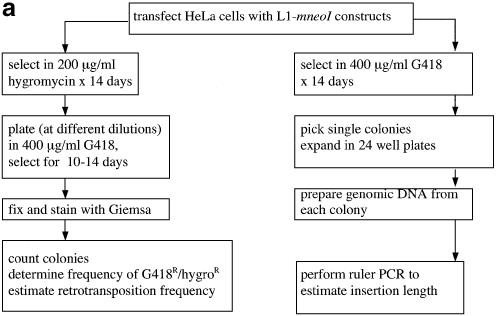
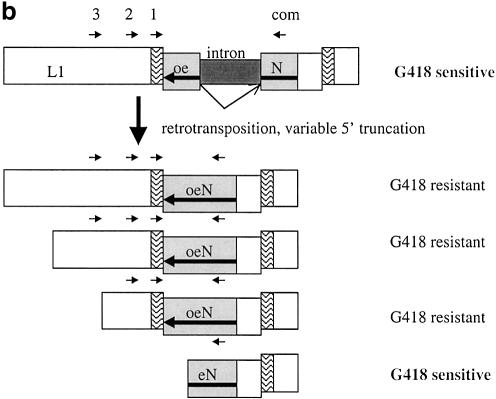
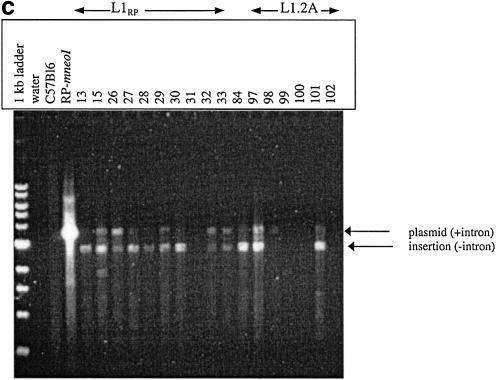
(a) Overview of assay system to measure L1 retrotransposition frequency and insertion length in cultured cells. Transfection and selection of HeLa cells containing neomycin-tagged L1 constructs (L1-mneoI) were carried out as described previously (6,35). Two parallel assay systems were established to measure retrotransposition frequency and estimate L1 insertion length (see text). (b) L1 retrotransposition cassette and predicted results for variable 5′ truncation of L1 insertions. When L1-mneoI undergoes retrotransposition, the intron in the anti-sense neomycin resistance gene (mneoI) is spliced out, yielding mneo and conferring resistance to the antibiotic G418. The parental L1-mneoI construct is sensitive to G418 because the intron cannot be spliced out of the anti-sense mneoI transcript. Similarly, insertions that lack the full-length spliced neomycin resistance gene are sensitive to G418. The map positions of the ruler PCR primers are as follows: 3 kb primer (position 3961 in L1), 2 kb primer (4819), 1 kb primer (5892) and com (reverse primer in the mneoI gene, beyond the intron, position 7819). Predicted amplicon sizes for spliced products using the primer com and primers 3, 2 and 1, are 2949, 2091 and 1018 bp, respectively. Insertions of different lengths will be positive for one or more of these ruler PCRs. Sequence analysis of the spliced and unspliced amplicons was performed to validate each of the ruler PCRs. (c) Three kilobase ruler PCR on G418R HeLas transfected with L1-mneoI constructs. Three kilobase ruler PCR was performed using primers 3 kb and com. The plasmid, L1-mneoI, gives a band at 3858 bp, while the L1-mneo insertion, L1-mneo, gives a band at 2949 bp. Lanes contain a 1 kb ladder (New England Biolabs), distilled water, C57Bl6 (wild-type mouse DNA), RP-mneoI (retrotransposition plasmid) and genomic DNA samples from G418R clones transfected with L1RP (clones 13, 15, 26–33), L1RP with L1.2RT (clone 84) or L1.2A (clones 97–102).