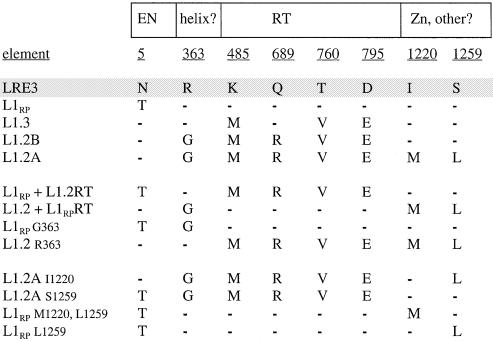
Figure 2.
Alignment of ORF2 sequences of active human L1 elements surveyed for insertion length and frequency. Shown in the boxed regions are the known functional domains of the L1 ORF2 protein. These include the endonuclease domain (EN) and the RT domain. In between the EN and RT domains is a region that contains alternating basic and uncharged residues that may form alpha helices spanning amino acids 313–365. Residue 363 is located in a putative alpha helix. In the very 3′ end of the ORF2p, beyond the putative zinc knuckle motif, is a region with intermittently placed basic residues that includes residues 1220 and 1259. L1 constructs are given in the first column. Amino acid residues, numbered from the N- to C-terminal portion of the ORF2p, are underlined. Amino acid sequences are aligned relative to LRE3 (shaded). Residues that are identical to the corresponding residue in LRE3 are denoted by dashes. Five human L1 elements were studied: LRE3, L1RP, L1.3, L1.2B and L1.2A. Derivative constructs include RT domain swaps (L1RP with the RT domain of L1.2 and vice versa) and various site-directed mutants (see text).