Abstract
Aquaporin-4 (AQP4), the most abundant water channel in the brain, plays a central role in water homeostasis, neuronal activity, and migration of astrocytes in the central nervous system. Recent studies have demonstrated that AQP4 is a target of an autoantibody specifically detected in an autoimmune neurologic disease called neuromyelitis optica. Here we have generated a monoclonal antibody (MAb) against the C-terminal region of AQP4 using a baculovirus expressing mouse AQP4 as an immunogen. This antibody (clone E5206) recognized both human and mouse AQP4s in a denaturing condition and was able to precipitate AQP4 from cell lysates of CHO cells stably expressing AQP4. Western blot analysis using deletion mutants revealed that the epitope was located within a region between Asp303 and Leu320 in the C-terminal tail of AQP4. Although clone E5206 could not be used for immunostaining when cells or tissues were fixed with 4% paraformaldehyde or 10% formalin, it could be used when cells were fixed with 10% trichloroacetic acid and when a formalin-fixed tissue section was pretreated with antigen-retrieval reagents. This MAb can be a valuable tool for analysis of AQP4 in a variety of physiological and pathophysiological contexts, in human tissues and organs as well as in rodent models, both in vitro and in vivo.
Introduction
Aquaporin-4 (AQP4) is a water channel expressed in the brain, spinal cord, kidney, lung, skeletal muscle, stomach, and small intestine.(1,2) In the brain, it is a predominant water channel mainly expressed in the subpial and perivascular endfeet of astrocytes(3) and plays a central role in water homeostasis, neuronal activity, and migration of astrocytes in the central nervous system.(4–6) AQP4 functions as a tetramer consisting of two main isoforms, M1 and M23, produced by the difference in the transcriptional start sites; the longer isoform, M1, has an extra 22 amino acids at the N terminus of the M23 isoform.(7–9) These two isoforms have opposing roles in forming an orthogonal array of particles (OAPs), a unique feature of AQP4, whose physiological roles remain to be elucidated; while M23 tends to form OAPs, M1 tends to disrupt them.(10)
Recent studies have demonstrated that an autoantibody found in patients with the autoimmune neurologic disease neuromyelitis optica (NMO), which is characterized by optic neuritis and acute myelitis,(11) recognizes the extracellular domains of AQP4.(12,13) Loss of astrocytic markers, including AQP4, is evident. Complement-dependent disruption of astrocytes prior to demyelination has been suggested in the tissue of human patients as well as in rodent models, both in vitro and in vivo.(14–20)
AQP4 is a highly conserved membrane protein; therefore, it is difficult to establish a good MAb against AQP4. The only exception is clone 3/D2 raised against a synthetic peptide corresponding to amino acids 301–318 of rat AQP4.(21) Unexpectedly, this MAb recognizes human AQP4 (hAQP4) but does not do so for mouse or rat AQP4 (mAQP4 or rAQP4, respectively). Here, we established a new MAb, E5206, using mice with AQP4-null background in combination with baculovirus expressing mAQP4 as an immunogen, which can be used for a wide range of techniques, including Western blot analysis, immunoprecipitation, and immunostaining of both human and mouse cells and tissues.
Materials and Methods
Hybridoma preparation
Monoclonal anti-AQP4 antibodies were established according to the previous report.(22) In brief, the cDNA encoding mAQP4 M23 isoform was inserted into a pBlueBac4.5 plasmid transfer vector (Invitrogen, Carlsbad, CA) to produce budded baculovirus (BV) expressing mAQP4 M23. gp64 transgenic mice crossed with AQP4-null mice (acc. no. CDB0758K, www.cdb.riken.jp/arg/mutant%20mice%20list.html)(23) were immunized intraperitoneally with the BV expressing mAQP4 M23 in PBS in the presence of pertussis toxin. The fusion of NS-1 myeloma cells was carried out using standard methodology. After screening by flow cytometry and an enzyme-linked immunosorbent assay (ELISA) using CHO cells stably expressing mAQP4 M23, as well as by Western blotting using lysate derived from a membrane fractions of mouse cerebella, clone E5206 (IgG1) was obtained.
Plasmid construction
As reported recently, AQP4 M23 isoform can be translated from the AQP4-M1 mRNA through a leaky scanning mechanism.(24) Thus, to express M1 isoform exclusively, cDNAs encoding hAQP4 and mAQP4 M1, in which Met23 was changed to Leu (M23L-hAQP4 M1 and M23L-mAQP4 M1, respectively) were constructed by a QuickChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) using primers 5'-GTGTACCAGAGAGAACATCCTGGTGGCTTTCAAAGGGGTC-3' and 5'-GACCCCTTTGAAAGCCACCAGGATGTTCTCTCTGGTACAC-3' for M23L-hAQP4 M1; and 5'-CTGCAGTAGAGAGAGCATCCTGGTGGCTTTC-3' and 5'-GAAAGCCACCAGGATGCTCTCTCTACTGCAG-3' for M23L-mAQP4 M1.
To identify the epitope of E5206, two deletion mutants of mAQP4 △303–323 and △321–323 were constructed by PCR using oligonucleotides 5'-CCGCGGTCAAATCACATGCACCACTC-3' and 5'-CCGCGGTCACAATACCTCTCCCGAAGAGTC-3', respectively, as antisense primers and 5'-GGATCCTATACCGGTGCCAGCATGAATCCAGC-3' as a sense primer. Obtained PCR products were subcloned into pGEM-T vector (Promega, Madison, WI). After confirmation of the sequence, the fragments were excised with SacI and SacII followed by insertion into the corresponding region of wild-type mAQP4 M1 cDNA subcloned into a pIRES2-EGFP mammalian expression vector (Clontech Laboratories, Mountain View, CA).
Cell culture and transfection
CHO cells were maintained in Ham's F12 nutrient mixture supplemented with 10% fetal bovine serum and 50 U/mL penicillin and 50 μg/mL streptomycin. Cells seeded onto 60-mm dishes (1×105 cells/dish) were transiently transfected with AQP4 and its derivatives inserted into the pIRES2-EGFP, using Lipofectamine and Plus reagents (Invitrogen) according to the manufacturer's instructions. At 48 h after transfection, cells were lysed with a buffer containing 20 mM Tris-Cl (pH 7.4), 1 mM EDTA, 1% Triton X-100 and Complete™ protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, IN). Thirty μg of cellular protein was subjected to Western blot analysis as described previously,(25) using MAb E5206 (1:20 for supernatant of hybridoma culture), rabbit polyclonal anti-AQP4 C-terminal domain (1:1000; Sigma, St. Louis, MO), monoclonal anti-green fluorescent protein (1:500; clone mFX75, Wako Pure Chemical Industries, Osaka, Japan), or polyclonal rabbit anti-actin (1:5000, Sigma) as primary antibodies. Secondary antibodies used were HRP-conjugated goat anti-mouse and goat anti-rabbit antibodies (Sigma).
Immunoprecipitation
Immunoprecipitation was performed as described previously,(26) with slight modification. In brief, confluent monolayers of CHO cells stably expressing either M23L-mAQP4 M1 or mAQP4 M23 seeded onto 60-mm dishes were lysed with 250 μL Blue Native (BN)-buffer, vortexed, and centrifuged at 20,000 g for 5 min at 4°C. Supernatants were collected and incubated in the presence of 10 μg of either E5206 or monoclonal anti-hAQP4 extracellular domain antibody clone C9401(27) on a mechanical rotator overnight at 4°C. The next day, 20 μL of pre-washed nProtein A Sepharose 4 Fast Flow beads (GE Healthcare, Waukesha, WI) were added to the samples. To isolate the immunocomplexes, the samples were centrifuged at 11,000 g for 1 min at 4°C, and the beads were washed four times with washing buffer. mAQP4 was eluted by adding 20 μL of 2× Laemmli buffer at 37°C for 30 min and subjected to Western blotting using the rabbit polyclonal anti-AQP4 C-terminal domain (1:1000; Sigma). One-twentieth (12.5 μL) of each supernatant was used as an input.
Immunofluorescent staining and immunohistochemistry
CHO cells stably expressing either M23L-mAQP4 M1 or mAQP4 M23 isoform were fixed with either 4% paraformaldehyde (PFA) or 10% trichloroacetic acid (TCA).(28) After washing with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS, followed by blocking with 0.1% BSA in PBS. Binding of the MAb to AQP4 was visualized with Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen).
For immunofluorescent microscopy of mouse tissues, wild-type and AQP4-null mice were anesthetized with 40 mg/kg sodium pentobarbital and perfused through the left cardiac ventricle with 0.9% saline followed by 4% neutral buffered PFA. Cerebral cortices, cerebella, and kidneys were removed and post-fixed in 4% neutral buffered PFA followed by serial dehydration in 20% and 30% sucrose solutions. Organs were embedded in Tissue Tek OCT compound. Ten-mm frozen sections were prepared using a CM3050S cryostat (Leica Microsystems, Wetzlar, Germany). After antigen retrieval in 0.01 M sodium citrate buffer, tissues were blocked with 10% normal goat serum and stained with 1 mg/mL E5206 followed by Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen). All animal experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals of Keio University School of Medicine (09084-4).
For immunohistochemistry of human brain sections, sections from the parietal lobes of normal controls (n=3) were used. First, the paraffin sections on slides were immersed in xylene for 5 min three times; then they were immersed in 100% ethanol, 95% ethanol, and 90% ethanol for 5 min each. After washing with distilled water, we washed the slides three times with phosphate buffer saline (PBS). Non-specific binding was blocked with 10% goat serum for 15 min at room temperature. In addition, antigen retrieval was performed in citrate buffer (Diva Decloaker, Biocare Medical, Concord, CA) for 5 min at 100°C, where applicable. The slides were covered with primary antibodies—anti-AQP4 antibody (E5206, diluted at 1:20 for supernatant of hybridoma culture) or anti-AQP4 antibody (H-80, diluted at 1:500; Santa Cruz Biotechnology, Santa Cruz, CA)—and incubated for 8 h at 4°C. Then, after washing, the slides were incubated with an HRP-conjugated anti-mouse EnVision system (Dako, Carpinteria, CA) for 1 h at room temperature followed by staining with diaminobenzidine hydrochloride (DAB). The slides were counterstained with hematoxylin and mounted with VectaMount (Vector Labs, Burlingame, CA). This use of human specimens was approved by the ethical committee of the Tohoku University Graduate School of Medicine (no. 2011-74).
Results
Establishment of E5206
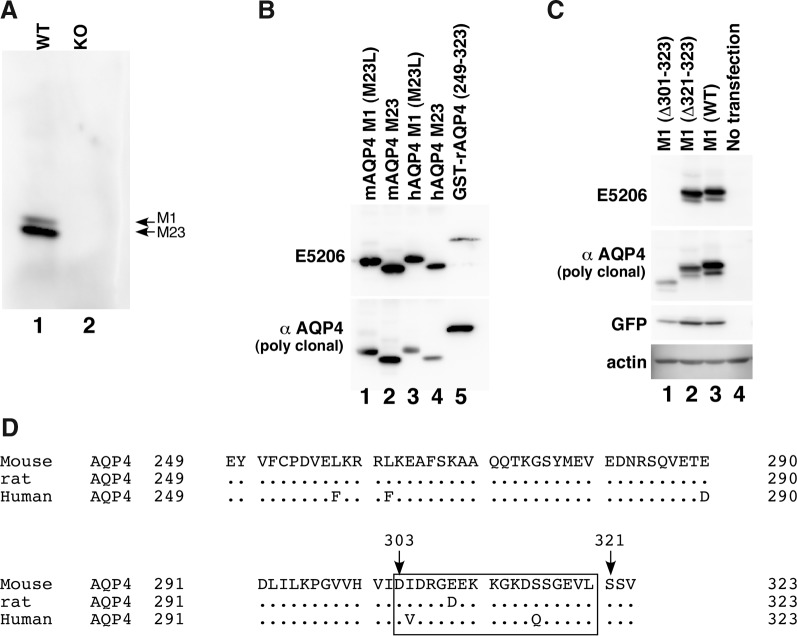
To circumvent immunological tolerance, BALB/c mice with AQP4-null background were immunized with baculovirus expressing mAQP4. One of the obtained clones, E5206 (IgG1), specifically recognized both the M1 and M23 isoforms of AQP4 in a lysate of mouse cerebellar membrane fraction in a denaturing condition (Fig. 1A). To examine the specificity of E5206, cDNA encoding either mouse or human AQP4 M1 (M23L) or M23 isoform inserted into pIRES2-EGFP was transiently transfected into CHO cells. Lysates of the cells were subjected to Western blotting using E5206 compared with commercially available rabbit polyclonal antibody against the C-terminal domain of rAQP4 (Sigma). As shown in Figure 1B, E5206 recognized both hAQP4 and mAQP4, regardless of the difference in isoforms (Fig. 1B, lanes 1–4). E5206 also recognized GST-fusion protein containing a peptide corresponding to Glu249-Val323 of rAQP4, which is the immunogen to raise Sigma's antibody (Fig. 1B, lane 5), indicating that the epitope for E5206 is located within the intracellular C-terminal domain of AQP4.
FIG. 1.
Establishment of MAb against the C-terminal domain of AQP4. (A) Western blotting of lysates of a cerebellar membrane fraction derived from either wild-type (lane 1) or AQP4-null (lane 2) mouse using conditioned medium of a hybridoma clone E5206 (1:20). (B) Western blotting of lysates from CHO cells transiently transfected with M23L-mAQP4 M1 (lane 1), mAQP4 M23 (lane 2), M23L-hAQP4 M1 (lane 3), or hAQP4 M23 (lane 4). The GST-fused rAQP4 C-terminal domain, which is the immunogen to raise the polyclonal anti-AQP4 C-terminal domain antibody from Sigma, was also electrophoresed (lane 5). (C) Western blotting of CHO cells transiently transfected with mAQP4 M1 (△301–323, lane 1), mAQP4 M1 (△321–323, lane 2), or wild-type mAQP4 M1 (lane 3). Lysate of CHO cells without transfection was also electrophoresed as a negative control (lane 4). (D) Alignment of protein sequences from Glu249 to Val323 of human, rat, and mouse AQP4, which corresponds to the region used as an immunogen to develop polyclonal rabbit anti-AQP4 C-terminal antibody derived from Sigma. Amino acids conserved among these three species are represented as dots. Amino acids immediately after the truncated site of two deletion mutants are indicated by arrows. The predicted epitope for E5206 is indicated with a box.
Epitope of E5206
To determine the epitope of E5206, two deletion mutants of mAQP4 M1 (Fig. 1D) were transiently expressed in CHO cells, and lysates of the cells were examined to determine whether E5206 would recognize them by Western blot analysis. While E5206 recognized mAQP4 M1 (△321–323), which lacks the last three amino acids of mAQP4 (Fig. 1C, lane 2), it did not do so for mAQP4 M1 (△303–323), which lacks the last 21 amino acids (Fig. 1C, lane 1), thus indicating that the epitope is located between Asp303 and Leu320 in the C-terminal tail of AQP4.
Immunoprecipitation
Next we examined whether E5206 is applicable to immunoprecipitation. Lysates derived from CHO cells stably expressing mAQP4 M23 or M23L-mAQP4 M1 were incubated with E5206 followed by precipitation with nProtein A Sepharose 4 Fast Flow beads. As shown in Figure 2, although C9401, a MAb specific to hAQP4 extracellular domains,(27) did not precipitate mAQP4 (lanes 2, 5), E5206 efficiently precipitated both M1 and M23 isoforms of mAQP4 (lanes 6, 3, respectively). Consistent with the result of Western blot analysis (Fig. 1B), E5206 also precipitated hAQP4 from CHO cells stably expressing hAQP4 M23 isoform (data not shown).
FIG. 2.
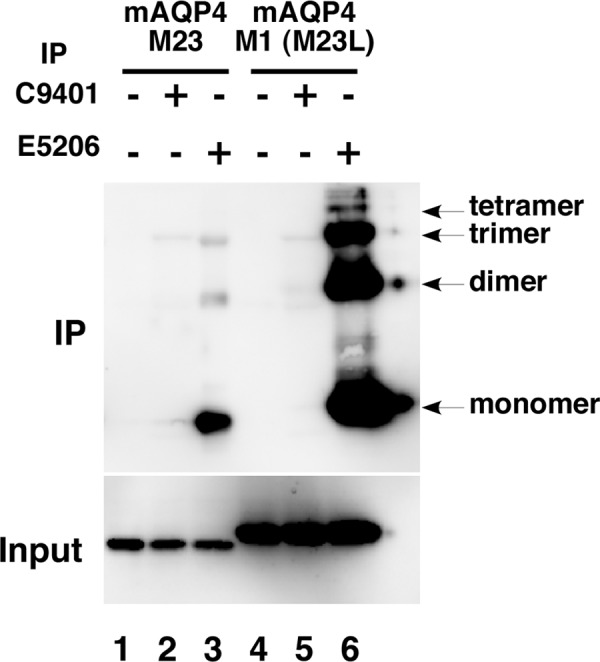
Immunoprecipitation of mAQP4 by E5206. Lysates from CHO cells stably expressing mAQP4 M23 (lanes 1–3) and M23L-mAQP4 M1 (lanes 4–6) were incubated without (lanes 1, 4) or with either MAb C9401 (lanes 2, 5) or E5206 (lanes 3, 6), followed by precipitation with nProtein A Sepharose 4 Fast Flow beads. Precipitates (IP, upper panel) and input (lower panel) were subjected to Western blot analysis using rabbit polyclonal anti-AQP4 C-terminal domain antibody.
Immunostaining
We also addressed whether E5206 can be used for immunostaining of AQP4-expressing cells and tissues. First we stained CHO cells stably expressing either M23L-mAQP4 M1 or mAQP4 M23, which were the same cell lines used for immunoprecipitation, with E5206. When cells were fixed with 4% PFA, they were not stained with the MAb, even in the presence of membrane permeabilization with Triton X-100 (Fig. 3A). However, when cells were fixed with 10% TCA, both cells expressing M23L-mAQP4 M1 and mAQP4 M23 were clearly stained with E5206 in a dose-dependent manner (Fig. 3B).
FIG. 3.
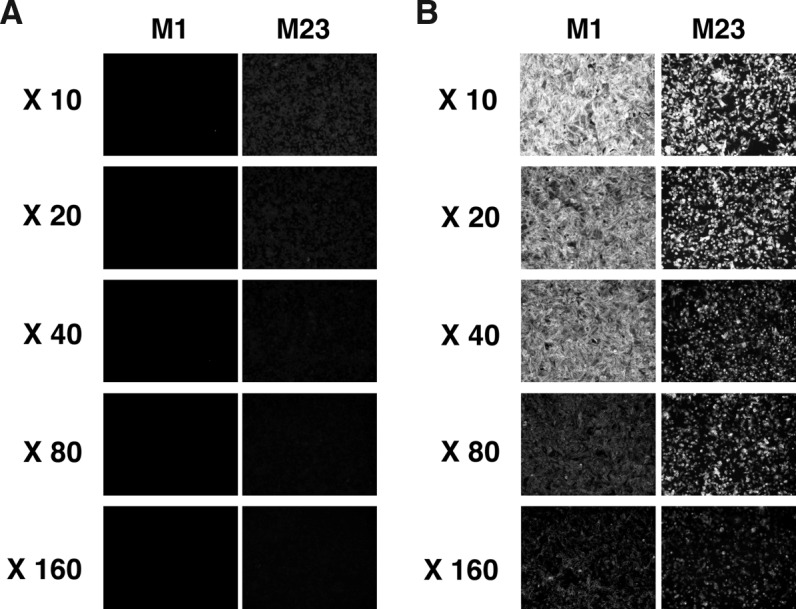
Immunofluorescent staining of CHO cells stably expressing mAQP4 with E5206. (A) CHO cells stably expressing either M23L-mAQP4 M1 (M1) or mAQP4 M23 (M23) isoform were fixed with 4% PFA (A) or 10% TCA (B) and permeabilized with 0.1% Triton X-100 in PBS. Conditioned medium of the hybridoma clone E5206 was serially diluted and added to the cells.
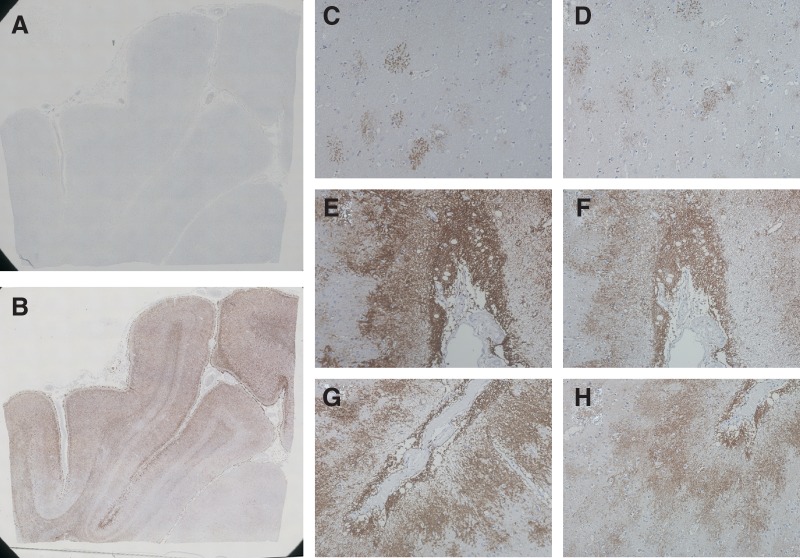
E5206 can also be used for paraffin-embedded human tissue samples. As expected from the result of immunofluorescent staining of AQP4-expressing CHO-cell lines, in the absence of antigen retrieval, E5206 did not work when cortex or subcortical white matter of human parietal lobes were stained (Fig. 4A). However, after treatment with antigen retrieval reagent, E5206 clearly stained AQP4 (Fig. 4B) with the same pattern as commercially available rabbit polyclonal antibody against the C-terminal 80 amino acids (244–323) of human AQP4 (H-80) in several regions in the human brain (Fig. 4C–H). Similarly, E5206 specifically stained the perivascular endfeet of astrocytes in the cerebral cortices (Fig. 5A, B) and in the cerebellar granular layers (Fig. 5C, D), as well as the kidney collecting ducts (Fig. 5E, F) in antigen-retrieval reagent-treated mouse frozen sections.
FIG. 4.
Immunohistochemistry of the paraffin section of human brain with E5206. Cortex and subcortical white matter from human parietal lobes was stained with E5206 in the absence (A) or presence (B-H) of antigen retrieval. White matter (C, D), cortex adjacent to a sulcus (E, F), and a region surrounding a sulcus (G, H) stained with either E5206 (C, E, G) or rabbit polyclonal anti-AQP4 C-terminal domain antibody H-80 (D, F, H) are shown magnified (×200).
FIG. 5.
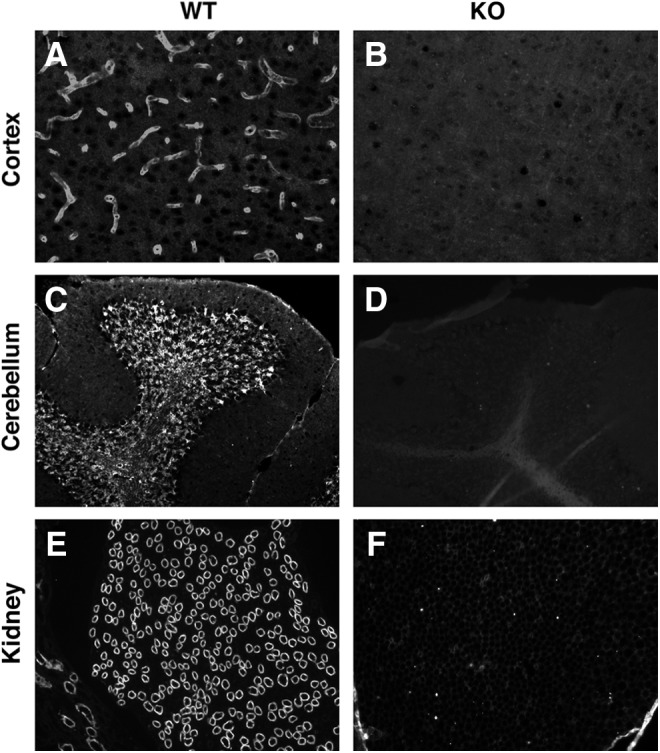
Immunofluorescent staining of mouse tissue with E5206. Frozen sections with antigen retrieval of cerebral cortices (A, B,×200), cerebella (C, D,×100), and kidneys (E, F,×100) derived from wild-type (A, C, E) or AQP4-null (B, D, F) mice were stained with E5206.
Discussion
In this study, we have developed a MAb against the C-terminal region of mAQP4 designated as clone E5206. E5206 recognized not only mAQP4 but also hAQP4, and therefore could be used for a wide variety of techniques, including Western blot analysis, immunoprecipitation, and immunostaining of cells and tissues.
Previously, Nagy and colleagues developed a similar MAb (clone 3/D2) against synthetic peptide corresponding to amino acids 301–318 of rAQP4.(21) Epitope mapping of E5206 (Fig. 1B) demonstrated that its epitope was found in almost the same region as the immunogen peptide of 3/D2. Therefore, it is likely that the amino acid sequence of this region is suitable as an antigen. In addition, this region is the most divergent in the C-terminal domain of AQP4 among species (Fig. 1D); therefore, 3/D2 could be developed by immunization of wild-type mice with the synthetic peptides. Nevertheless, despite the fact that 3/D2 was raised against the peptide with a rat sequence, it does not recognize rAQP4 or mAQP4. In contrast to the strategy of Nagy's group, we succeeded in developing a MAb recognizing both hAQP4 and mAQP4 by using AQP4-null mice.
In immunostaining of cells and tissues with E5206, formaldehyde-based fixation did not lead to good results. But antigen retrieval or using another fixative such as TCA greatly improved the performance of E5206. Two possibilities may explain this phenomenon. One is modification of the epitope by reactions of three lysine residues located within it with formaldehyde as well as following intramolecular cross-linkage between intracellular domains, which affects the antibody binding. The other is cross-linkage of AQP4-associated proteins, whose binding site overlaps with the epitope for E5206, with the C-terminal domain of AQP4, which masks the epitope and interferes with antibody binding. Since AQP4 has a motif for PSD95-Discs large-ZO1 (PDZ)-binding at the C-terminus, it is highly likely that proteins containing the PDZ-domain are associated with the C-terminal region of AQP4. One candidate, α-syntrophin, regulates expression level and subcellular localization of AQP4.(29) Thus, E5206 can be a valuable tool to examine the function of AQP4-associated protein by microinjection of this MAb as a competitor into cells endogenously expressing AQP4 and its associated proteins.
Acknowledgments
The authors thank Drs. Keisuke Horiuchi and Kazuki Yuasa for help with making frozen sections; Dr. Dovie Wylie for expert assistance; and all members of the Department of Pharmacology, School of Medicine, Keio University for their cooperation. This work was supported by the Global Center of Excellence Program for Humanoid Metabolomic Systems Biology of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; Grant-in-Aid for Scientific Research B (General) of MEXT of Japan (21390061); the Japan New Energy and Industrial Technology Development Organization (NEDO, P08005); Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects; the NFAT project of the New Energy and Industrial Technology Development Organization (NEDO, P06009), Japan; Keio Gijuku Academic Development Funds; and Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) (22590940).
Author Disclosure Statement
The authors have no financial conflicts to declare.
References
- 1.Jung JS. Bhat RV. Preston GM. Guggino WB. Baraban JM. Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa H. Ma T. Skach W. Matthay MA. Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994;269:5497–5500. [PubMed] [Google Scholar]
- 3.Nielsen S. Nagelhus EA. Amiry-Moghaddam M. Bourque C. Agre P. Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verkman AS. Binder DK. Bloch O. Auguste K. Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Tait MJ. Saadoun S. Bell BA. Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2007;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Zelenina M. Regulation of brain aquaporins. Neurochem Int. 2010;57:468–488. doi: 10.1016/j.neuint.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Yang B. Ma T. Verkman AS. cDNA cloning, gene organization and chromosomal localization of a human mercurial insensitive water channel: evidence for distinct transcriptional units. J Biol Chem. 1995;270:22907–22913. doi: 10.1074/jbc.270.39.22907. [DOI] [PubMed] [Google Scholar]
- 8.Lu M. Lee MD. Smith BL. Jung JS. Agre P. Verdijk MAJ. Merkx G. Rijss JPL. Deen PMT. The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Natl Acad Sci USA. 1996;93:10908–10912. doi: 10.1073/pnas.93.20.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T. Yang B. Verkman AS. Gene structure, cDNA cloning, and expression of a mouse mercurial-insensitive water channel. Genomics. 1996;33:382–388. doi: 10.1006/geno.1996.0214. [DOI] [PubMed] [Google Scholar]
- 10.Furman CS. Gorelick-Feldman DA. Davidson KGV. Yasumura T. Neely JD. Agre P. Rash JE. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci USA. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingerchuk DM. Lennon VA. Pittock SJ. Lucchinetti CF. Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 12.Lennon VA. Wingerchuk DM. Kryzer TJ. Pittock SJ. Lucchinetti CF. Fujihara K. Nakashima I. Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 13.Lennon VA. Kryzer TJ. Pittock SJ. Verkman AS. Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misu T. Fujihara K. Kakita A. Konno H. Nakamura M. Watanabe S. Takahashi T. Nakashima I. Takahashi H. Itoyama Y. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 15.Roemer SF. Parisi JE. Lennon VA. Benarroch EE. Lassmann H. Bruck W. Mandler RN. Weinshenker BG. Pittock SJ. Wingerchuk DM. Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 16.Hinson SR. Pittock SJ. Lucchinetti CF. Roemer SF. Fryer JP. Kryzer TJ. Lennon VA. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita M. Nakatsuji Y. Moriya M. Okuno T. Kumanogoh A. Nakano M. Takahashi T. Fujihara K. Tanaka K. Sakoda S. Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. NeuroReport. 2009;20:508–512. doi: 10.1097/wnr.0b013e32832776f4. [DOI] [PubMed] [Google Scholar]
- 18.Sabater L. Giralt A. Boronat A. Hankiewicz K. Blanco Y. Llufriu S. Alberch J. Graus F. Saiz A. Cytotoxic effect of neuromyelitis optica antibody (NMO-IgG) to astrocytes: an in vitro study. J Neuroimmunol. 2009;215:31–35. doi: 10.1016/j.jneuroim.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H. Bennett JL. Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol. 2011;70:943–954. doi: 10.1002/ana.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saadoun S. Waters P. Bell BA. Vincent A. Verkman AS. Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy G. Szekeres G. Kvell K. Berki T. Németh P. Development and characterisation of a monoclonal antibody family against aquaporin 1 (AQP1) and aquaporin 4 (AQP4) Pathol Oncol Res. 2002;8:115–124. doi: 10.1007/BF03033720. [DOI] [PubMed] [Google Scholar]
- 22.Saitoh R. Ohtomo T. Yamada Y. Kamada N. Nezu J-I. Kimura N. Funahashi S-I. Furugaki K. Yoshino T. Kawase Y. Kato A. Ueda O. Jishage K-I. Suzuki M. Fukuda R. Arai M. Iwanari H. Takahashi K. Sakihama T. Ohizumi I. Kodama T. Tsuchiya M. Hamakubo T. Viral envelope protein gp64 transgenic mouse facilitates the generation of monoclonal antibodies against exogenous membrane proteins displayed on baculovirus. J Immunol Methods. 2007;322:104–117. doi: 10.1016/j.jim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Ikeshima-Kataoka H. Abe Y. Abe T. Yasui M. Immunological function of aquaporin-4 in stab-wounded mouse brain in concert with a pro-inflammatory cytokine inducer, osteopontin. Mol Cell Neurosci. 2013;56:65–75. doi: 10.1016/j.mcn.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A. Pisani F. Nicchia GP. Svelto M. Frigeri A. Evidences for a leaky scanning mechanism for the synthesis of the shorter M23 protein isoform of aquaporin-4: implication in orthogonal array formation and neuromyelitis optica antibody interaction. J Biol Chem. 2010;285:4562–4569. doi: 10.1074/jbc.M109.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe Y. Ikeshima-Kataoka H. Goda W. Niikura T. Yasui M. An astrocyte-specific enhancer of the aquaporin-4 gene functions through a consensus sequence of POU transcription factors in concert with multiple upstream elements. J Neurochem. 2012;120:899–912. doi: 10.1111/j.1471-4159.2012.07652.x. [DOI] [PubMed] [Google Scholar]
- 26.Pisani F. Mastrototaro M. Rossi A. Nicchia GP. Tortorella C. Ruggieri M. Trojano M. Frigeri A. Svelto M. Identification of two major conformational aquaporin-4 epitopes for neuromyelitis optica autoantibody binding. J Biol Chem. 2011;286:9216–9224. doi: 10.1074/jbc.M110.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki K. Abe Y. Iwanari H. Suzuki Y. Kikuchi T. Ito T. Kato J. Kusano-Arai O. Takahashi T. Nishiyama S. Ikehsima-Kataoka H. Tsuji S. Arimitsu T. Kato Y. Sakihama T. Toyama Y. Fujihara K. Hamakubo T. Yasui M. Establishment of monoclonal antibodies against the extracellular domain that block binding of NMO-IgG to AQP4. J Neuroimmunol. 2013;260:107–116. doi: 10.1016/j.jneuroim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K. Yonemura S. Matsui T. Tsukita S. Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. J Cell Sci. 1999;112:1149–1158. doi: 10.1242/jcs.112.8.1149. [DOI] [PubMed] [Google Scholar]
- 29.Neely DJ. Amiry-Moghaddam M. Ottersen OP. Froehner SC. Agre P. Adams ME. Syntrophin-dependent expression and localization of aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]