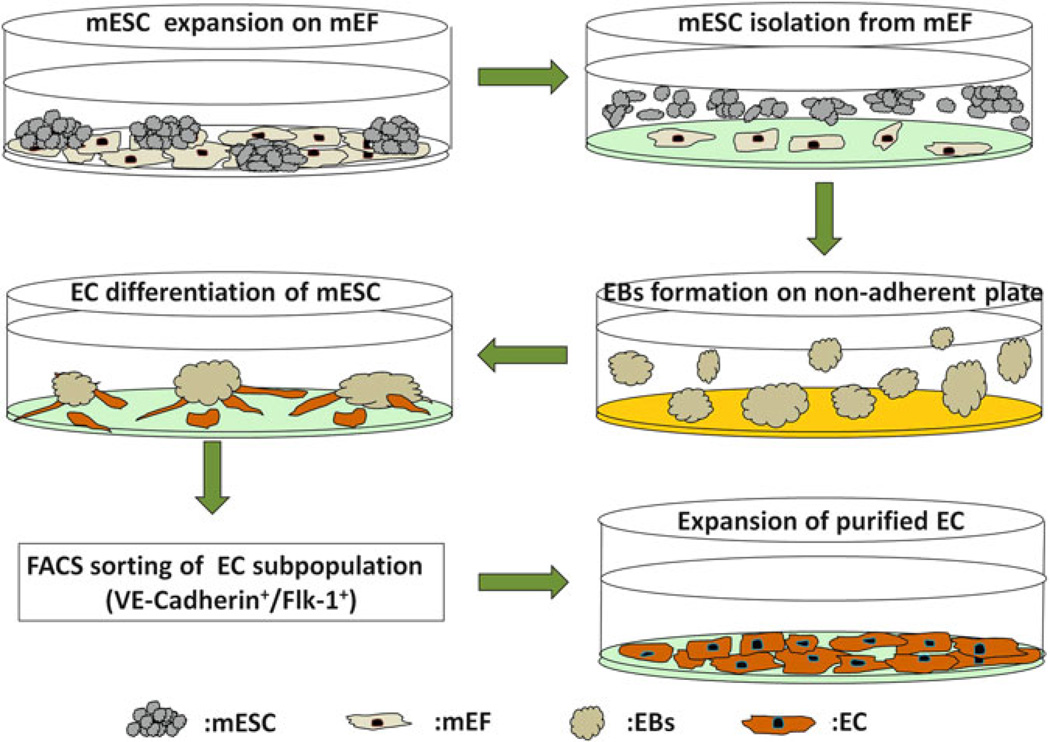
Fig. 4.
Schematic diagram demonstrating all steps during EC differentiation of ESCs and expansion of purified cells. The mESCs expanded on the mEF feeder layer are first subcultured on 0.1 % gelatinized plates to separate mEFs from the mESCs before EB formation. The non-adherent mESCs are then transferred to non-adherent plates coated with Poly-HEMA; the loss of stromal contact and withdrawal of Leukemia inhibitory factor (LIF) induce formation of 3D EBs. After 5 days of culture, the optimally sized EBs are switched to EC differentiation medium and are transferred to gelatinized plates the next day. EBs are cultured on the gelatinized plates in EC differentiation medium for up to 7 additional days. FACS sorting is then used to purify the emerging endothelial subpopulation expressing the endothelial markers (Flk-1+ and VE-Cadherin+). Purified sorted ECs can then be further expanded by culturing on gelatinized coated plates.