Fig. 1. GLUT1 homology model.
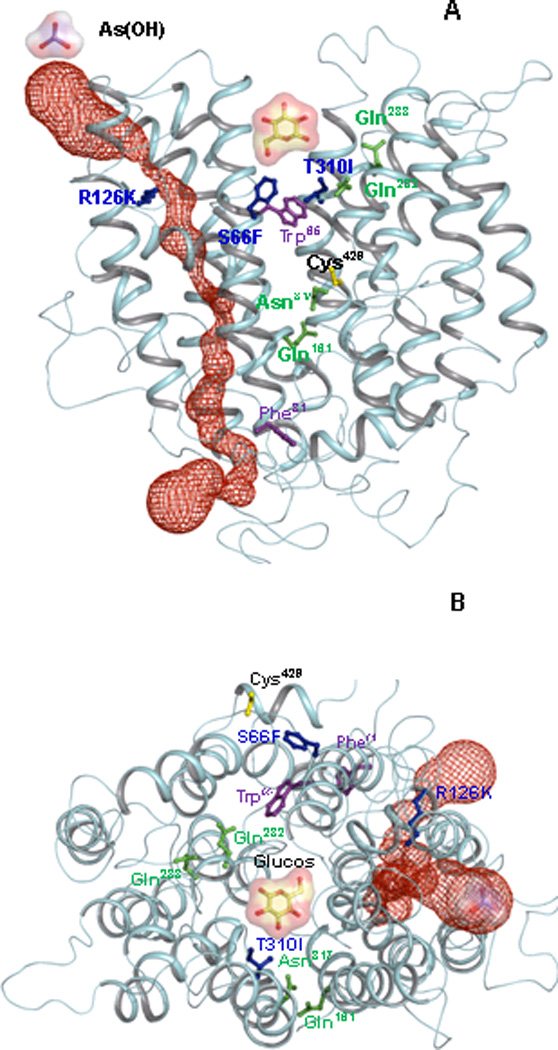
Helices are rendered as Cα worms, colored cyan. (A) View from within the plane of the membrane, with the exofacial side at top and cytosollic side at bottom. In this open form, the glucose binding site is shown as a cavity, (B) Exofacial view of the open form of GLUT1. Selected residues are shown in ball and stick: Gln161, Gln282, Gln283 and Asn317 are shown in green. The GLUT1-DS mutants S66F, R126K, T310I are shown in blue. Cys429, the site of Hg(II) binding, is shown in yellow. Trp65 and Phe81, which are the extra- and intracellular forskolin binding sites, respectively, are shown in magenta. The auxiliary (red) channel is shown as space filling representation. Glucose and As(OH)3 molecules are illustrated as ball-and-stick with molecular surface representations.
