FIGURE 3.
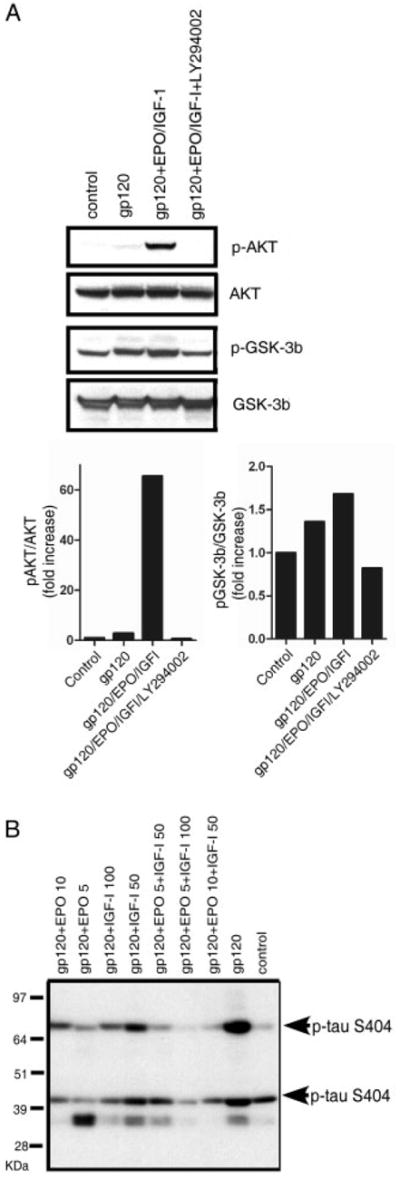
Erythropoietin (EPO)+insulin-like growth factor-I (IGF-I) activates the PI3K/Akt/glycogen synthase kinase (GSK)-3β pathway to counteract gp120-induced tau hyperphosphorylation. Western blots of cortical culture lysates following a 1-day exposure to 200pM gp120 are shown. (A) EPO+IGF-I treatment produced Akt phosphorylation and GSK-3β phosphorylation, whereas total Akt and GSK-3β remained constant. Pretreatment with 50μM of the PI3K inhibitor LY294002 completely abrogated Akt phosphorylation and largely blocked the phosphorylation of GSK-3β engendered by EPO+IGF-I. (B) Hyperphosphorylation of tau following exposure of cerebrocortical cultures to gp120. Antitau clone 5E2 (Upstate Biotechnology, Lake Placid, NY) was used to immunoprecipitate (IP) total tau protein from cortical cell culture lysates (this antibody brings down tau isoforms in the molecular weight range of ~45–70kDa). The IPs were then run on a 10% NuPage gel and probed with phospho-tau antibody (directed against ser404, which is known to represent tau hyperphosphorylation). In the blot, 2 isoforms of tau are seen to be phosphorylated at serine 404 (bands at ~45 and 70kDa). Tau phosphorylation increased after exposure to 200pM gp120, but was prevented by treatment with EPO+IGF-I.
