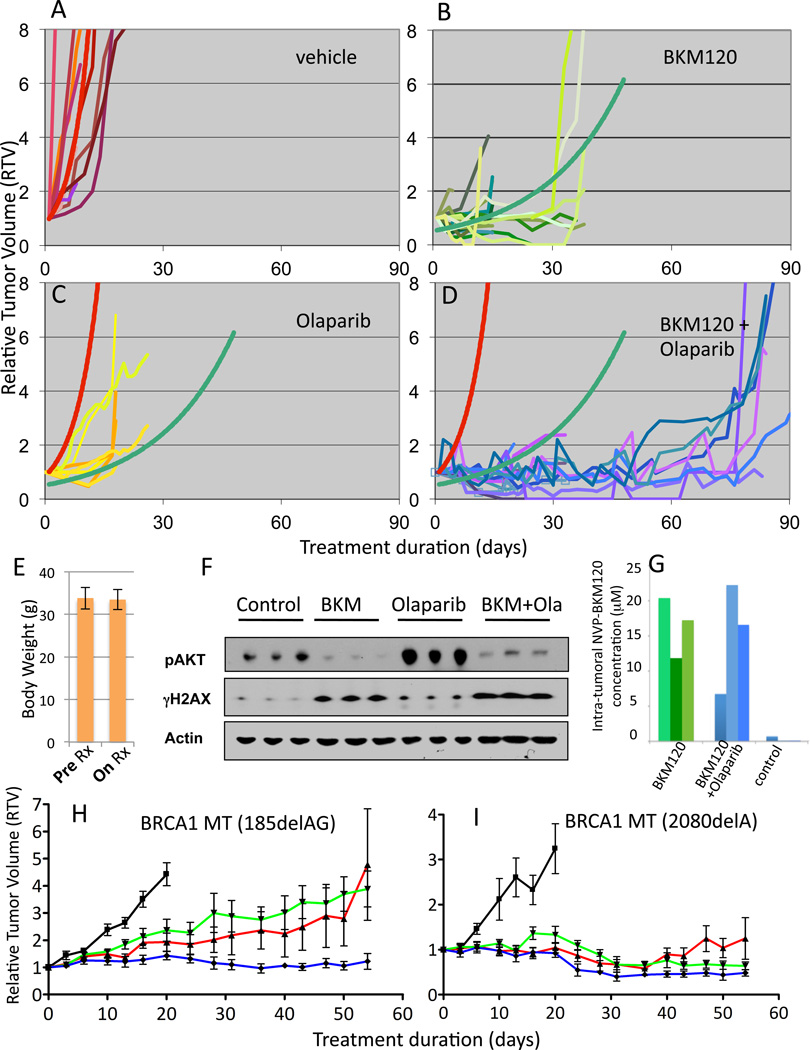
Fig. 6.
Anti-tumor efficacy of PI3K inhibitor NVP-BKM120 alone and in combination with Olaparib. A–D Tumor-bearing MMTV-CreBRCA1 p53+/− were treated with either vehicle control (A), NVP-BKM120 (B, 50 mg/kg/day (n=11) or 30 mg/kg/day (n=10)), Olaparib (C, 50 mg/kg/day (n=8)) or the combination of NVP-BKM120 and Olaparib (D, NVP-BKM 50 mg/kg/day+Olaparib 50 mg/kg/day (n=8) or NVP-BKM 30 mg/kg/day+Olaparib 50 mg/kg/day (n=7)) and tumor volumes were measured every 2–3 days using calipers. Trendlines for vehicle control (red curve) and NVP-BKM120 treatments (green curve) were calculated using all data points to determine best fit. The functions of the best-fit curves were used to determine tumor doubling times for all three treatment modalities and controls. E, Stable body mass with PI3K-inhibitor and PARP-inhibitor treatments Mice were weighed before and after treatments. F, G. Target inhibition and pharmacokinetics in vivo. Tumor tissues harvested from animals treated with NVP-BKM120 (30 mg/kg/day) alone or in combination with Olaparib (50 mg/kg/day) as indicated were harvested 3 hours after the last treatment and subjected to immunoblotting with antibodies against Actin, p-AKT and γH2AX (F) or lysed and subjected to Mass Spectrometry (G). For standards used see Materials and Methods and Fig. S5. H, I. Responses of human BRCA1 -related breast cancers implanted as xenotransplants into nude mice to NVP-BKM120, Olaparib or their combination. Breast cancer tissues from two patients, one with a 185delAG germline mutation (H) and the other one with a 2080delA germline mutation (I) were propagated as subcutaneous implants in nude mice. Tumors were allowed to grow to a size of 5 mm when mice were randomized to treatments with either vehicle control (black, —), NVP-BKM120 (red,  ), Olaparib (green,
), Olaparib (green,  )or their combination (blue,
)or their combination (blue,  ) (n=6 for each cohort, same dosing as in F). Tumor assessment with electronic calipers was done as described in Materials and Methods.
) (n=6 for each cohort, same dosing as in F). Tumor assessment with electronic calipers was done as described in Materials and Methods.