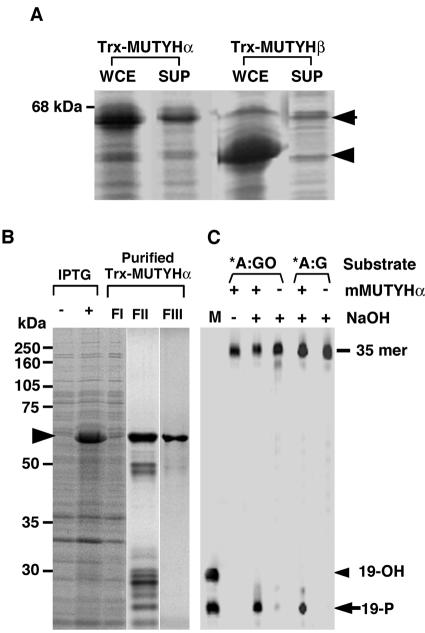
Figure 9.
Expression and purification of recombinant mMUYTH proteins. (A) The expression of recombinant mMUTYH proteins. E.coli cells transformed with pET32a-mMUTYHα and pET32a-mMUTYHβ were grown in the presence of IPTG, and harvested cells were disrupted with sonication as described in Materials and Methods. Whole cell extracts (WCE) or supernatant after centrifugation (SUP) were subjected to western blotting with anti-hMUTYH. (B) Purification of recombinant Trx-mMUTYHα. E.coli cells transformed with pET32a-mMUTYHα were grown in the presence (+) or absence (–) of IPTG, and supernatant fraction (FI) was prepared from the harvested cells. Trx-mMUTYHα protein was purified using cobalt beads (FII) followed by gel filtration (FIII), as described in Materials and Methods. Each fraction was analyzed by SDS–PAGE and the gel was stained with Coomassie Brilliant Blue. (C) Biochemical characterization of purified Trx-mMUTYHα. Duplex oligonucleotide substrates containing A:8-oxoG (*A:GO) and adenine:guanine (*A:G) pairs, in which the 5′-end of one strand carrying target base adenine (*A) was labeled with FAM, were incubated with (+) or without (–) the purified Trx-mMUTYHα protein (FIII), as described in Materials and Methods, and the reaction products were fractionated by gel electrophoresis with (+) or without (–) NaOH treatment. M, marker oligonucleotides whose 5′-end was labeled with FAM (19-OH and 19-P).