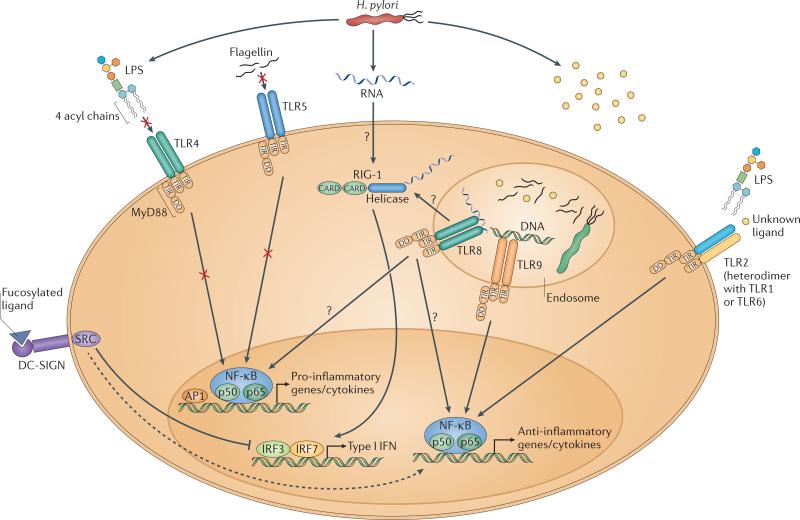
Figure 2. H. pylori subversion of innate immune recognition.
H. pylori harbors PAMPs that have evolved to evade detection by pro-inflammatory TLRs. H. pylori expresses tetra-acylated LPS, which is less bioactive than the hexa-acylated form typical of other Gram-negative pathogens due to specific lipid A modifications that prevent detection by TLR4. H. pylori flagella are not detected by TLR5 due to mutations in the TLR5 binding site of flagellin. The bacterium's DNA, as well as an as yet uncharacterized PAMP (and possibly H. pylori LPS) are detected by TLRs 9 and 2, respectively; these TLRS predominantly activate anti-inflammatory signalling pathways and anti-inflammatory IL-10 expression. 5‘ triphosphorylated RNA is detected by the RLR RIG-I, which activates the transcription factors IRF3 and IRF7 to induce type I IFN expression, and is potentially detected also by TLR8 in endosomes. H. pylori's fucosylated DC-SIGN ligands suppress activation of the signalling pathways downstream of this CLR and activate anti-inflammatory genes. Please note that not all depicted TLRs, RLRs and CLRs are necessarily expressed by the same cell type; only one generic cell type is shown here for simplicity. DD, death domain; TIR, Toll/Interleukin-1 receptor domain; CARD, caspase activation and recruitment domain; MyD88, myeloid differentiation primary response gene 88; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin; SRC, steroid receptor coactivator.