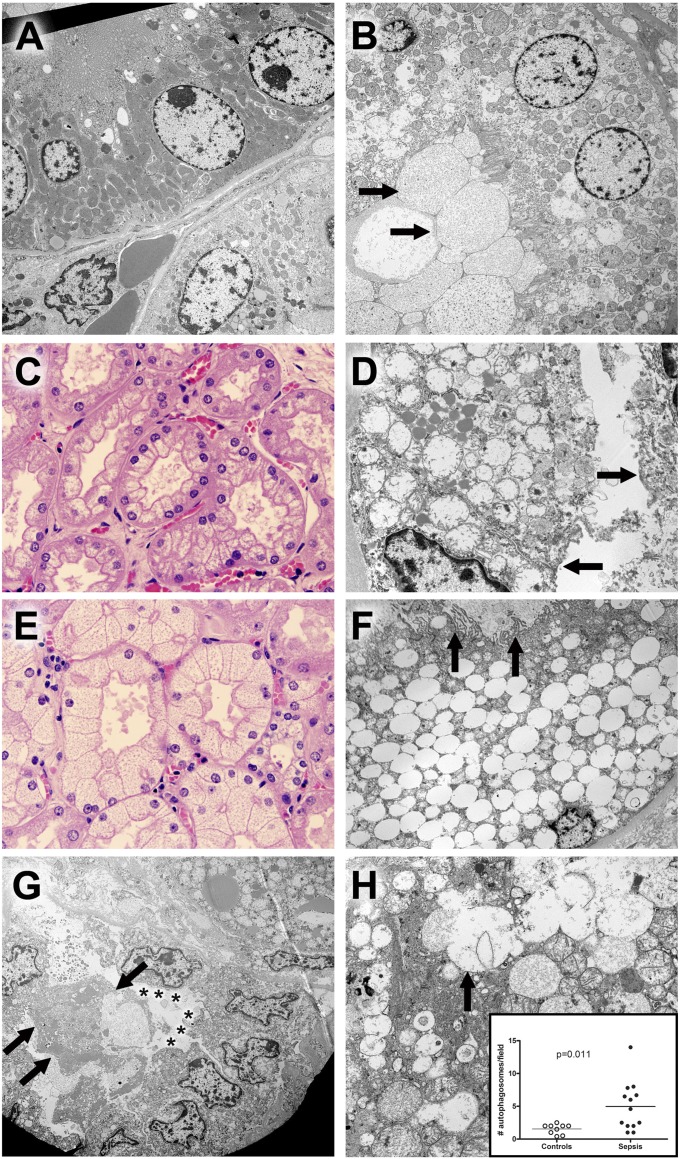
Figure 4.
Hematoxylin and eosin (H&E) and electron microscopic findings in kidney. (A) Ultrastructure of normal tubules from a control sample. A proximal tubule is shown in the upper half of this image. Cells are low columnar with uniform round nuclei. The apical membrane (upper left) comprises uniform microvilli that project into the lumen (not shown). Mitochondria are large, with relatively dark profiles. Few lysosomes are seen. In the lower half of the image, cells with fewer and smaller mitochondria are present. No microvillus brush border is present. These are distal tubular epithelial cells. Original magnification: ×3,070. (B) Proximal tubule epithelial damage in sepsis. In contrast to normal proximal tubular epithelium, the cytoplasm of these cells contains scattered dilated lysosomes, some with complex internal membrane structure, consistent with autophagy. Although the microvillus brush border is focally present, it is clear that this and other elements of apical membranes have been lost on some cells; in these areas, dilated organelles and cytoplasmic appear to be extruded into the lumen in a manner analogous to apocrine secretion, resulting in accumulation of membrane-bound cell debris (arrows in lower left hand corner). Original magnification: ×2,460. (C) Corticomedullary junction from a septic patient demonstrating coarse vacuolization of the proximal tubules. H&E staining. Original magnification: ×600. The corresponding electron microscopic image from this kidney is seen in adjacent view (4D). (D) Hydropic mitochondria in cells with coarse vacuolization. In this proximal tubule, epithelial cells contain enlarged, hydropic mitochondria with damaged crista. Apical membranes are damaged, with loss of the microvillus brush border (arrows). Lipid droplets are conspicuous. Original magnification: ×6,150. (E) Corticomedullary junction from a different septic patient showing isometric fine vacuolization of the proximal tubules. H&E staining. Original magnification: ×600. The corresponding electron microscopic image from this kidney is seen in an adjacent view (4F). (F) Dilated lysosomes in cells from septic kidney with isometric fine vacuolization. In this proximal tubule, epithelial cells are expanded by enlarged, focally fused membrane-bound structures. Absent obvious autophagic characteristics or evidence of mitochondrial breakdown, these are best interpreted as lysosomes. Relatively normal mitochondria and other cytoplasmic elements (as well as the nucleus) are displaced to the periphery of the cell. Apical membranes still retain some (but not all) microvilli (arrows), and cell debris (focally with features of autophagosomes) occupies the tubule lumen. Original magnification: ×2,460. (G) Damage and regeneration in proximal tubules. In the upper right hand corner of the image, a cell with hydropic mitochondria and large lipid droplets is present. In the lower half of the image, a proximal tubule with three important features is highlighted: 1) detachment and sloughing of a cell into the lumen (arrows) and 2) loss of the microvillus brush border (asterisks) in a series of cuboidal epithelial cells that also exhibit 3) clustering of nuclei. The latter feature has been suggested as a morphologic corollary of regenerating tubular epithelial cells. Original magnification: ×1,840. (H) Autophagy in septic tubular epithelium. In this image, relatively normal mitochondria are juxtaposed with dilated lysosomal structures, some of which (arrow) are fused with adjacent structures or contain membrane fragments (autophagosomes). Mitochondrial membrane damage is also apparent. Original magnification: ×6,150. Insert: Dot plot of average autophagosomes per 3,070× field in septic and control samples. Although nearly all samples had recognizable autophagic elements, the mean number per image was significantly increased in septic kidneys. Each dot represents an individual patient.