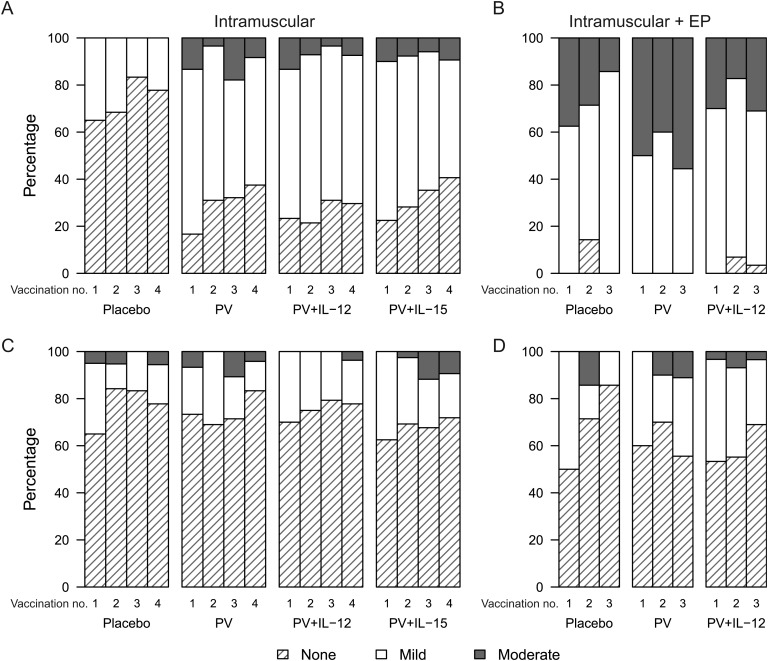
Figure 2.
Reactogenicity symptoms. Maximum severity of local pain or tenderness (A and B) and of systemic symptoms (C and D) following each study injection. A and C, Intramuscular administration. B and D, Intramuscular administration with electroporation. No severe symptoms were reported. Onset of a reaction was within the first 3 days following a study injection. Reactions were followed to resolution to determine the maximum severity. Systemic reactions included malaise and/or fatigue, headache, chills, myalgia, arthralgia, nausea, and vomiting. For intramuscular administration, the interleukin 15 (IL-15) 0.8-mg and 2-mg dose groups are combined because there were no differences in the reactogenicity profiles. Abbreviations: EP, electroporation; IL-12, interleukin 12; PV, PENNVAX-B DNA vaccine.