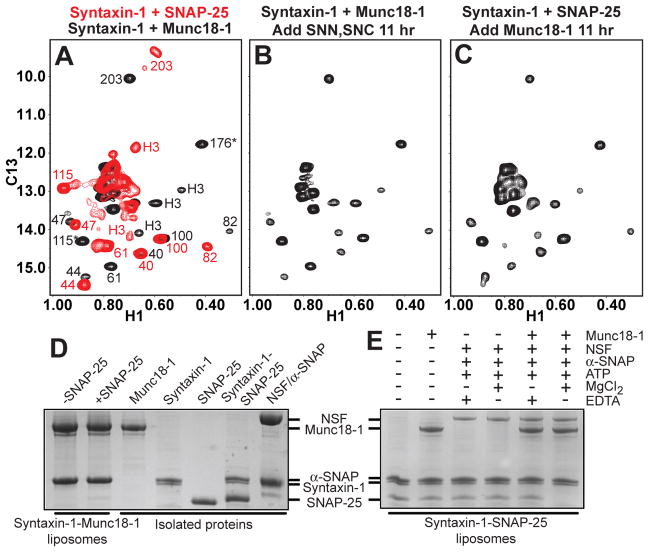
Figure 1.
Munc18-1 displaces SNAP-25 from syntaxin-1. (A-C) 1H-13C HMQC spectra of 2H-Ile-13CH3-syntaxin-1 bound to Munc18-1 (black) or to SNAP-25 (red) (A); initially bound to Munc18-1 and then incubated with the SNAP-25 SNARE motifs (SNN and SNC) for 11 hr (B); and initially bound to SNAP-25 and then incubated with Munc18-1 for 11 hr. In panel A, the available cross-peak assignments for the syntaxin-1-Munc18-1 complex are indicated in black and those for the syntaxin-1-SNAP-25 complex in red [for assignments, see ref. (13); H3 identifies the SNARE motif; * indicates tentative assignments]. Because of the 2:1 stoichiometry of the syntaxin-1-SNAP-25 complex, the cross-peak of I203 is double. (D) Proteoliposomes containing co-expressed syntaxin-1-Munc18-1 complex were incubated with SNAP-25 or buffer, co-floatation assays were performed, and the top fraction was analyzed by SDS-PAGE and Coomassie Blue staining (left two lanes). (E) Proteoliposomes containing syntaxin-1 were incubated with Munc18-1, SNAP-25, NSF, α-SNAP, ATP, Mg2+ and/or EDTA as indicated. Co-floatation assays were then performed and the results analyzed by SDS-PAGE and Coomassie Blue staining. The five lanes on the right of panel D show loading controls with soluble proteins.