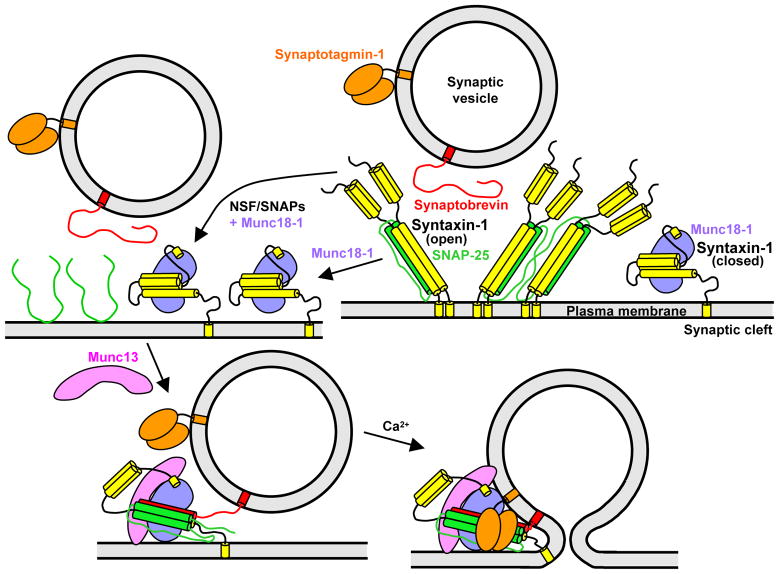
Figure 5.
Model of synaptic vesicle fusion integrating the function of eight major components of the release machinery. In the upper right panel, syntaxin-1 (yellow) is shown in a closed conformation bound to Munc18-1 and in an open conformation bound to SNAP-25. To illustrate the likely heterogeneity of syntaxin-1-SNAP-25 heterodimers, two complexes with 2:1 or 4:2 stoichiometries are shown, but larger complexes bridged by SNAP-25 (not shown) are also likely to exist. The model postulates that the syntaxin-1-SNAP-25 heterodimers are converted to syntaxin-1-Munc18-1 complexes (upper left), and that Munc13 helps to open syntaxin-1 and to orchestrate trans-SNARE complex assembly together with Munc18-1, leading to a partially assembled SNARE complex that remains bound to Munc18-1 and Munc13 (lower left). This state, which may correspond to that of primed synaptic vesicles and cannot be disassembled by NSF-SNAPs, serves as the substrate for synaptotagmin-1-Ca2+ to trigger fast synaptic vesicle fusion (lower right). The arrangement of Munc18-1, Munc13 and synaptotagmin-1 with respect to the SNARE complex is unknown, but is drawn to suggest the possibility that the three proteins may bind simultaneously to the SNARE complex and may also help to bridge the two membranes to help inducing fusion. The interaction of synaptotagmin-1 with the SNARE complex may occur before Ca2+ influx (not shown in the lower left panel for simplicity).