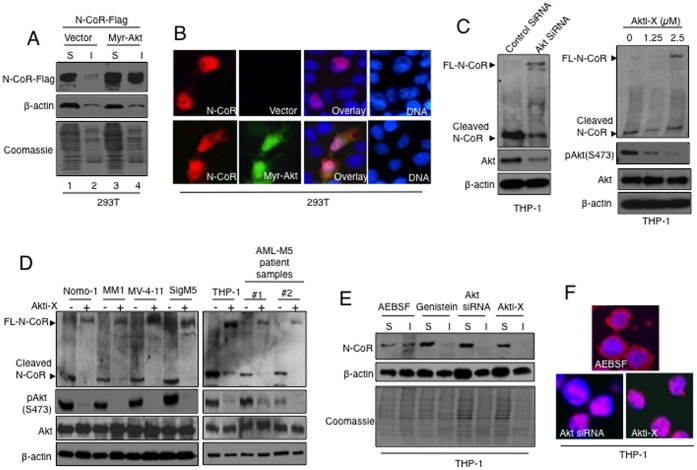
Figure 3. Akt promotes N-CoR misfolding.
A, Relative solubility/insolubility of flag tagged N-CoR expressed with the constitutively active Akt (myr-Akt) in 293T cells was determined by protein solubility assay. Soluble (S) and insoluble (I) fractions were separated by high-speed centrifugation and N-CoR levels in each fraction were determined by western blotting assay using Flag antibody. The constitutively active Akt (myr-Akt) caused a significant increase in insoluble N-CoR protein levels. The relative solubility/insolubility of β-actin in each fraction served as a control. The level of total protein in each fraction was determined by coomassie blue staining. B, Subcellular distribution of N-CoR-Flag (red fluorescence) in 293T cells transfected with flag-tagged N-CoR with or without the constitutively active GFP tagged myr-Akt (green fluorescence) was determined by confocal microscopy. N-CoR-Flag was targeted to the cytosol when co-expressed with GFP-myr-Akt, while N-CoR-Flag expressed with empty vector was localized mainly in the nucleus. C, Inhibition of Akt leads to N-CoR stabilization. N-CoR and Akt level in THP- 1 cells treated with Akt or control siRNA was determined by western blotting with the respective antibodies (left panel). Levels of N-CoR, pAkt and Akt in THP-1 cells treated in a dose dependent manner with Akti-X, the commercially available specific inhibitor of Akt, was similarly determined (right panel). D, Therapeutic inhibition of Akt leads to N-CoR stabilization in primary and secondary AML-M5 cells. Levels of N-CoR, pAkt and Akt in AML-M5 cells (left panel) and human primary AML-M5 cells (right panel) treated in a dose dependent manner with Akti-X was determined. E, Native N-CoR conformation is rescued by Akt abrogation. Relative solubility/insolubility of N-CoR protein in Akt siRNA or Akti-X treated THP-1 cells was determined by protein solubility assay. Soluble (S) and insoluble (I) fractions of treated THP-1 cells were separated by high speed centrifugation and N-CoR level in each fraction was determined by western blotting assay using N-CoR antibody. THP-1 cells treated with AEBSF or genistein was used as controls. The relative solubility/insolubility of β-actin in each fraction served as an experimental control. The level of total of protein in each fraction was determined by coomassie blue staining. F, Subcellular distribution of N-CoR (red signal) in THP-1 cells treated with AEBSF at 200 µM, Akt siRNA or Akti-X at 2.5 µM was determined by confocal microscopy.