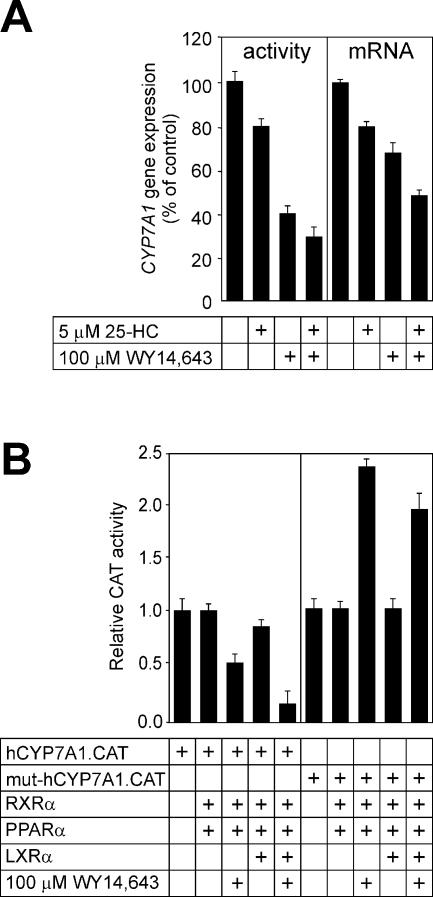
Figure 7.
Regulation of CYP7A1 gene expression by fibrates and oxysterols in HepG2 cells. (A) HepG2 cells were incubated for 24 h with 5 µM 25-HC, 100 µM WY 14,643, a combination of 25-HC and WY 14,643, or vehicle (0.1% dimethyl sulfoxide for WY 14,643 and 0.1% ethanol for 25-HC), as indicated, in medium containing 5% lipoprotein-deficient serum. The cyp7a enzyme activity was measured in prepared microsomes (left) and the cyp7a mRNA abundance was estimated by reverse transcription (RT)–PCR (right). The glyceraldehyde-6-phosphate dehydrogenase mRNA abundance was used to normalize cyp7a mRNA abundance. Values are expressed relative to controls (no ligands added). (B) The wild-type human CYP7A1 promoter–reporter gene chimera (hCYP7A1.CAT, 1 µg) (left) or the mutant derivative carrying the multibase substitution at Site I (mut.hCYP7A1.CAT, 1 µg) (right) were introduced into cells along with PPARα (0.5 µg), LXRα (0.5 µg) or RXR (0.5 µg) expression plasmids, as indicated. The cells were treated with WY 14,643, where indicated, in medium containing 5% lipoprotein-deficient serum.