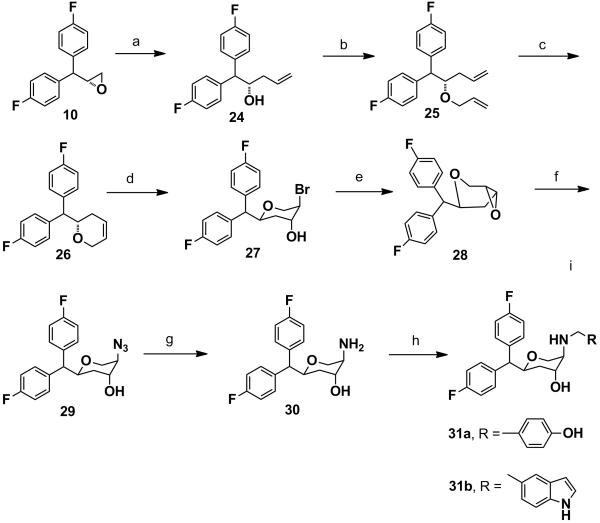
Scheme 6.
Synthesis of 31a-31b: Reagents and conditions: (a) vinylmagnesium bromide, CuI, anhyd. THF, −78 °C-rt, 24 h, 93%. b) NaH, allyl bromide, anhyd. DMF, 1.5 h, 0 °C-rt, 95%. c) 1st generation Grubb’s catalyst, anhyd. benzene, reflux, 2 h, 96%. d) N-bromoacetamide, Dioxane-H2O, 0 °C-rt, 4 h, 82%. e) 20% NaOH, Dioxane, 0 °C-rt, 30 min, 90%. f) NaN3, anhyd. DMF, 80 °C, 24 h, 96%. g) Pd/C, MeOH, H2, 30 psi, 98%. h) RCHO, NaCNBH3, 1,2-dichloroethane, AcOH, overnight.