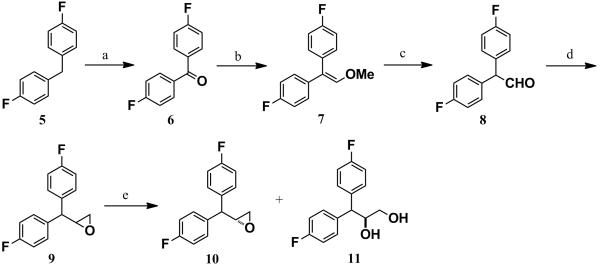
Scheme 3.
Synthesis of 10 and 11: Reagents and Conditions: a) KMnO4, CH3CN, 0 °C-rt, 99%. b) MeOCH2PPh3 +Br−, anhyd. THF, anhyd. NaNH2, 0 °C-rt, 97%. c) Conc. H2SO4, glacial AcOH, rt, 30 min, 97%. d) trimethylsulfoxonium iodide, anhyd. DMSO, NaH, rt, 1 h, then 60 °C, 2 h 95%. e) (R,R)-(−)-N,N/-bis(3,5-di-tert-butylsalicylidine)-1,2-cyclohexane diaminocobalt (Jacobsen’s catalyst), H2O, THF, over 99% ee and 48% yield for both 10 and 11.