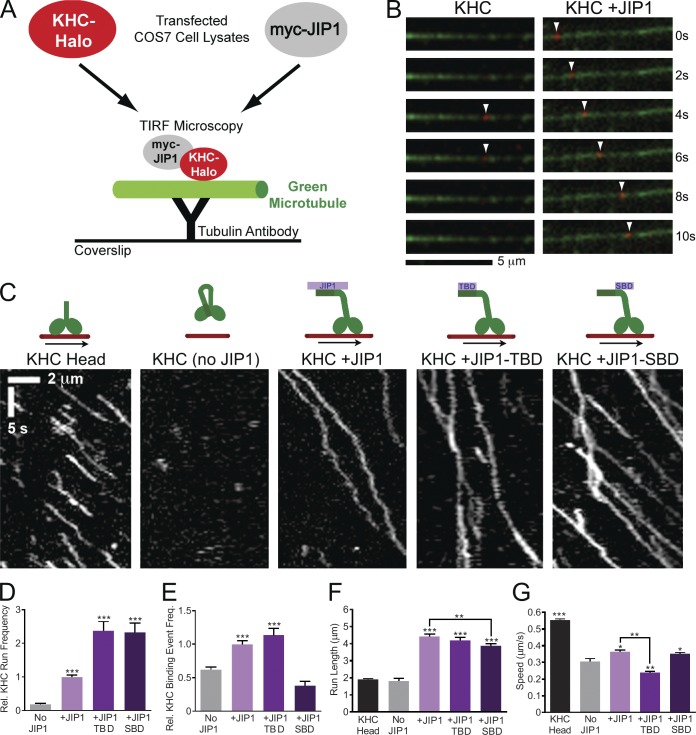
Figure 3.
JIP1 binding relieves KHC autoinhibition in in vitro TIRF motility assays. (A) Schematic of in vitro TIRF motility assay. Lysate from COS7 cells transfected with KHC-Halo and incubated with red fluorescent TMR ligand was combined with lysate from cells expressing myc-JIP1 constructs and applied to flow chambers containing green fluorescent microtubules, which were immobilized on glass coverslips with anti-tubulin antibody. KHC-Halo motility was imaged using a TIRF microscope. (B) Time-lapsed images acquired from a flow chamber containing KHC-Halo (red) lysate alone (left) show a brief non-motile binding event (arrowheads) to a microtubule (green). Images from a flow chamber containing KHC-Halo and myc-JIP1 lysates (right) show processive movement (arrowheads) along the microtubule. (C) Representative kymographs show activation of KHC-Halo by full-length JIP1, JIP1-TBD, or JIP1-SBD. 100 total frames (∼33 s) are shown. (D) Addition of full-length JIP1, JIP1-TBD, and JIP1-SBD increases the run frequency of full-length KHC-Halo. The absolute number of runs per 10 µm of microtubule was normalized to the KHC-Halo +JIP1 condition for each experiment. (D–G) Data represent three or more independent experiments per condition (n = 52–181 microtubules and n = 109–758 runs) and statistical comparisons were made relative to the KHC-Halo alone (no JIP1) condition unless otherwise indicated. (E) Addition of full-length JIP1 or JIP1-TBD increases the relative frequency of non-motile microtubule-binding events by full-length KHC-Halo. The number of non-motile binding events per 10 µm microtubule length was normalized relative to the KHC-Halo +JIP1 condition for each independent experiment. (F) Addition of full-length JIP1, JIP1-TBD or JIP1-SBD increases KHC-Halo run lengths. (G) Addition of full-length JIP1 or JIP1-SBD but not JIP1-TBD increases speed of KHC-Halo runs. Error bars show the mean ± SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001.