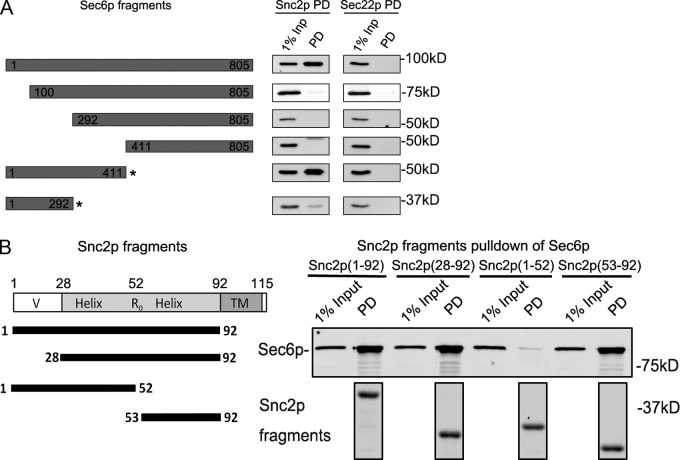
Figure 6.
The N terminus of Sec6p binds to the end of the Snc2p SNARE domain. (A) Full-length or truncated regions of Sec6p were expressed in bacteria as 6xHis-tagged constructs. The 1–411 and 1–292 fragments (indicated by asterisks) of Sec6p were coexpressed with a fragment of Sec8p (1–236) that binds to them and helps to maintain their solubility. The purified Sec6p fragments or Sec6p–Sec8p complexes (50 nM final concentration) were mixed with Snc2p-GST or Sec22p-GST (50 nM final concentration) in a 1-ml binding assay. The bound proteins were recovered on glutathione beads and analyzed by immunoblot analysis. The region from 1–411 of Sec6p is sufficient for Snc2p binding, whereas both the regions from 1–100 and 292–411 are required. (B) Fragments of Snc2p fused to GST were expressed in bacteria, purified, and mixed at 80 nM (final concentration) with 50 nM (final concentration) His-tagged Sec6p in a 1-ml binding assay. After precipitation of the GST-tagged constructs with glutathione beads (shown in the bottom panel by Coomassie staining of a gel in which intervening lanes have been removed for presentation purposes), the recovery of Sec6p was determined by immunoblot. The region from 53–92 of Snc2p is both necessary and sufficient for Sec6p binding, whereas the N-terminal 1–52 fragment shows no detectable binding. Relevant lanes from the corresponding Coomassie gel are shown in the bottom panel.