Figure 1.
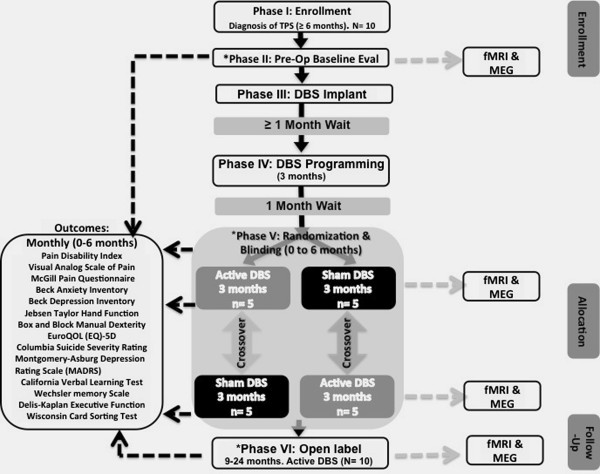
Study design. Flowchart of the study design based on specified CONSORT clinical trial guidelines [23]. Categories defined in CONSORT guidelines are marked on the right margin of the figure. *Time points when neurological, neuropsychological pain or neuropsychological cognitive evaluations (Table 1) will be conducted. During phase V, fMRI and MEG evaluations will be conducted at 2 and 5 months after randomized intervention allocation. During the open-label phase, follow-up assessments are conducted at month 9, 12, 18 and 24 for outcomes listed above and at month 12 and 24 for fMRI and MEG. TPS, thalamic pain syndrome; DBS, deep brain stimulation; fMRI: functional magnetic resonance imaging; MEG: magneto-encephalography; EuroQOL (EQ-5D), Euro Quality of Life scale.
