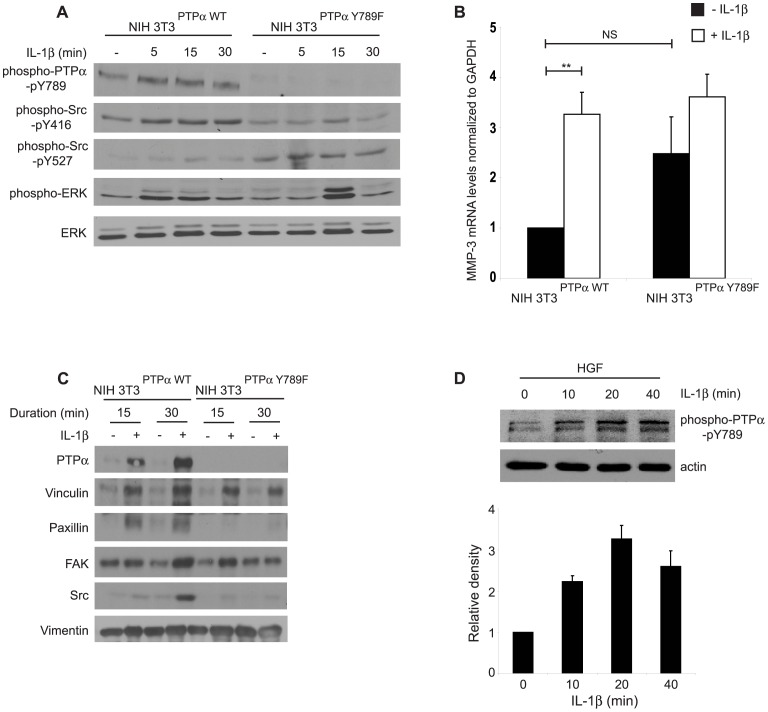
Figure 7. Effect of catalytic and adaptor functions of PTPα in IL-1β signaling.
(A) Whole cell lysates collected from NIH3T3 cells genetically modified to over-express wild-type PTPα (NIH3T3PTPα) or unphosphorylatable PTPα-Y789F mutant (NIH3T3Y789F) were plated on fibronectin and treated with or without IL-1β (40 ng/mL) for the time points indicated were immunoblotted for phospho-PTPα-pY789, phospho-Src-pY416, phospho-Src-pY527, phospho-ERK and ERK. (B) Same cells as above were treated with or without IL-1β (40 ng/mL; 4–5 hours) and analyzed by quantitative RT-PCR as described in Methods. Data are mean±S.E.M. of fold differences relative to the non-treated controls of NIH3T3PTPα in MMP-3 mRNA levels normalized to GAPDH mRNA levels from three independent experiments. (C) The cells described above in panels B and C, were plated on PLL for 4 hours, incubated with FN-coated magnetite beads and treated with vehicle or with IL-1β (40 ng/mL for 15 or 30 minutes). Magnetically-isolated bead-associated proteins were immunoblotted for PTPα, vinculin, paxillin, FAK, Src and vimentin. (D) Whole cell lysates prepared from human gingival fibroblasts grown on FN in the presence or absence of IL-1β (40 ng/mL) for varying time points as indicated, were immuno-blotted for phospho-PTPα-pY789 and actin. **p<0.01, NS: Not significant.