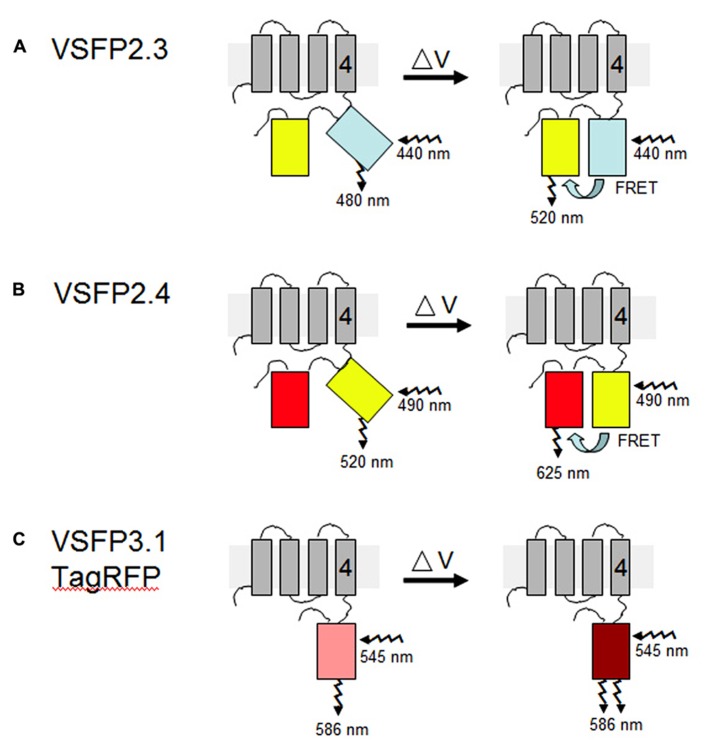
FIGURE 2.
VSFP probes of membrane potential. Different variants of VSFPs contain a combination of a voltage-sensing domain and either a pair of donor and acceptor fluorescent proteins for FRET-based monitoring or a single fluorescent protein for intensity-based recording. One fluorescent protein is attached to the fourth transmembrane segment (gray rectangles, nr. 4) of the C. intestinalis voltage sensor-containing phosphatase (Ci-VSP), which comprises transmembrane motifs S1 to S4 that coordinately operate as the voltage-sensing domain. (A,B) In the two FRET-based systems, VSFP2.3 and VSFP2.4, illumination with the excitation wavelength of the donor protein results in fluorescence primarily at the donor wavelength under resting conditions (left). A change in the membrane potential (δV) allows energy transfer between the two fluorescent proteins, perhaps by aligning them more favorably, such that increased fluorescence of the acceptor protein is observed concomitantly with decreased fluorescence of the donor protein (right). In VSFP2.3, the donor and acceptor fluorescent proteins are mCerulean and citrine, respectively. In VSFP2.4, the donor and acceptor fluorescent proteins are mCitrine and mKate2, respectively (Akemann et al., 2010). The approximate excitation and emission wavelengths of these proteins are shown. (C) In the intensity-based probe, VSFP3.1TagRFP, a decrease in membrane potential leads to an enhancement of fluorescence intensity from the fluorescent protein, in this case monomeric TagRFP. The figure is based on previously published ones (Perron et al., 2012; Mutoh and Knoepfel, 2013).