Abstract
Background
Minimally invasive percutaneous plate fixation (MIPPF) has gained wide acceptance for treating periarticular fractures of the tibia. Despite the theoretical biological advantages of MIPPF, it is unclear whether these are offset by wound complications, union rate, and malalignment.
Questions/purposes
We therefore estimated the rate of union in tibial fractures treated by MIPPF, the rate of major and minor wound complications, occurrence of malunion, and level of function.
Methods
We retrospectively reviewed 80 patients with tibia fractures treated by MIPPF. Mean age of patients was 43 years (range, 18–74 years); there were 54 male and 26 female patients. Severe soft tissue injuries were seen in 16 patients. Patients were examined clinically for soft tissue condition, ROM, alignment, and determination of function (Lower Extremity Functional Score). Radiographs and CT were obtained to determine union and malunion. The minimum followup was 12 months (mean, 23 months; range, 12–48 months).
Results
Sixty-three patients (79%) had uneventful healing of the fractures. We identified minor wound complications in seven patients (9%). Delayed union occurred in three (4%) and nonunion in seven (9%); noticeable axial malalignment occurred in five (6%), whereas rotational deformities greater than 10° were revealed with the use of CT in 25% of cases.
Conclusions
MIPPF allowed uneventful healing and restoration of the preinjury level of function in most patients. However, complications occurred in a substantial number of patients, which needs attention when planning surgery.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Minimally invasive percutaneous plate fixation (MIPPF) has gained wide acceptance for treating periarticular fractures of the tibia [1, 3, 5, 9, 12, 16, 18, 21]. The theoretical advantages of the method include minimal additional damage to the soft tissues in the fracture area and preservation of blood supply to the fragments of fracture, thereby providing better conditions for indirect fracture healing with callus.
Despite substantial interest in this topic, our search in PubMed with key words “minimally invasive plate osteosynthesis tibia” resulted in only 23 English-language articles that contained clinical information. Most publications [3, 5, 9, 12, 16, 21] are based on small numbers of patients (usually 20–30). This resulted in high interobserver variability, and reported rates of wound complications vary from 0% to 5% [7, 9, 12, 16] to 14.3% to 16.7% [3, 11]; the rates of consolidation disturbances vary from 0% [16] to 31.5% [21]. One of the potential pitfalls of MIPPF remains malunion, also with reports showing widely varying rates from 0% to 36.8% [3, 7, 9, 12]. Thus, despite the theoretical biological advantages of MIPPF, it is unclear whether these are achieved without increasing wound complications or rates of union and malalignment.
To address the controversies in previously published data, we determined (1) the rate of union in tibial fractures treated by MIPPF; (2) the rate of major and minor wound complications; (3) occurrence of malunion; and (4) level of function in a relatively large group of 80 patients.
Patients and Methods
We used MIPPF to treat 116 patients with lower leg fractures from January 2004 to July 2011. During the study time, we treated a total of 652 patients with these fractures. Indications for MIPPF were fractures in which intramedullary nailing (IMN) was not indicated or possible: (1) periarticular fractures of the tibia; (2) narrow intramedullary channel (less than 8 mm on radiographs, which precluded use of available nails); and (3) implant in situ (previous plate fixation of the same tibia). Before 2006, IMN was not available in our hospital, so MIPPF was performed in five cases with tibia shaft fractures that would be otherwise treated with IMN. Contraindications for MIPPF were severe soft tissue injuries that precluded the use of internal fixation, failure of intraoperative closed reduction of fracture, and the possibility of IMN (since 2006 in our settings). The criteria for inclusion in the study were (1) the use of MIPPF for the treatment of tibia fractures; (2) available followup results in terms of 12 months or more; and (3) informed consent from the patient to take part in the study. We excluded 35 patients with (1) pure tibial plateau fracture without extension on diaphysis (n = 27); (2) incomplete followup less than 12 months (n = 4); and (3) refusal to take part in the study (n = 4). One patient with a concomitant pelvic fracture died 6 weeks after the trauma and surgery as a result of a pulmonary embolism. These exclusions left 80 patients, who were included in this retrospective study. Mean age of patients at the time of injury was 42.7 ± 13.0 years (range, 18–74 years); there were 54 male and 26 female patients. The minimum followup was 12 months (mean, 22.7 months; range, 12–48 months). All data until fracture healing were obtained from medical records and radiographs. For the last followup, patients were recalled specifically.
Isolated lower leg injuries were seen in 63 patients and another 17 had additional injuries of other segments. In 45 patients, the injuries were the result of a fall on the street; road traffic accidents occurred in 26, sport injuries in eight, and one presented with a gunshot open fracture. Fracture distribution according to AO-Müller classification [13] is shown (Table 1). Despite most of the fractures being classified as shaft fractures, there were just five cases with classical indications to IMN (treated before 2006 when this method was not available in our settings); in another 50 cases, the center of fracture was at the level of metadiaphyseal junction (the borderline for IMN). Open fractures were seen in 12 patients and included Gustilo-Andersen I fractures [8] in three patients; Gustilo II in four; IIIA in three; and IIIB in two patients. Tscherne II closed soft tissue injuries [19] were seen in three patients and Tscherne III (compartment syndrome) in one patient. The remaining 64 patients had no major soft tissue injuries.
Table 1.
Distribution of fractures according to AO-OTA classification*
| Classification | 41 | 42 | 43 |
|---|---|---|---|
| A | 3 | 9 | 7 |
| B | 2 | 28 | – |
| C | 6 | 18 | 7 |
* In all cases of 41B and 41C injuries included in the study, the extension of fracture on the tibia shaft or combination with tibia shaft fracture was seen.
Time of surgery depended on soft tissue condition: the first hours after the injury or 5 to 10 days later when skin wrinkles appeared and in severe Gustilo-Andersen III open fractures after healing of the wound. The mean interval between injury and definitive surgery was 13 ± 17 days (range, 0–99 days). In 24 patients, surgery was performed later than 2 weeks after the injury (among them in three patients at 45–47 days; in another two at 88 and 99 days). Most of these patients were transferred from other hospitals. Six patients from this subgroup had severe open fractures; initial fixation was achieved with external fixation, but definitive fixation was delayed until soft tissues allowed this (average time to MIPPF in this subgroup was 60 days). Only split-thickness skin grafts were used to close the wounds; no local or microsurgical flaps were used to close soft tissue defects in these patients.
Surgical procedures were performed by seven surgeons, but the senior surgeon (AAS) performed 65 cases (73%). In all cases, surgery was performed in the supine position, but the position of the lower leg differed according to the fracture location. All surgeries were performed with the use of an image intensifier. In proximal tibia fractures, a triangle support was used to provide knee flexion at 30° to 60°. In tibia shaft or distal tibia fractures, a bolster was used to provide light flexion on the knee and elevation of the lower leg on the table. This facilitated radiographic control in the lateral view. Fracture reduction was achieved manually in most cases (68 patients), in 11 cases an external fixator or distractor was used, and in one patient with concomitant femoral neck fracture, the reduction was achieved on a fracture table. An incision 3 to 4 cm long was performed at the level of proximal or distal metaphysis according to the location of fracture. In case of an intraarticular fracture, the reduction and fixation of joint fragments were performed as a first stage. Then the subcutaneous or submuscular tunnel was prepared with the use of an elevator for subsequent plate insertion. The plate was bent with pliers according to the contours of bone in the fracture site. After insertion of the implant, the position of bone fragments and plate was checked with an image intensifier. One screw was inserted in each main fragment and position of the bone fragments was checked again. Then fixation was completed with insertion of a planned number of screws. Usually three to four 4.5-mm conventional or 5.0-mm locked screws were considered sufficient. In case of metaphyseal plates, a minimum of four 3.5-mm locked screws were inserted in the distal tibial fragment. The choice of side of plate insertion, medial or lateral, was based on fracture type and location as well as on soft tissue condition. In proximal tibia fractures, medial position of the plate was chosen in 11 patients, lateral position in 15, and in one case both lateral and posteromedial plates were used. In shaft fractures, the medial position was used in 15 cases and lateral in five. In distal tibia fractures, the plate was located in the medial position in 29 patients, one plate on the lateral side in one, and two plates (both on medial and lateral sides) in two patients. Different types of plated were used: DCP and LC-DCP (4.5 mm in 47 cases [among them wide in six and narrow in 41]; Medbiotech, Minsk, Belarus) and LCP (in 33 [among them LCP 3.5 mm in four, LCP 5.0 mm wide in one, LCP 5.0 mm narrow in 12, LCP 5.0/3.5 mm in 16]; ChM, Bialystok, Poland, and Osteosynthes, Rybinsk, Russia). After fracture fixation was completed and final radiological evaluation of all components of fixation performed, the wounds were closed; if the joint was opened, an active surgical drain was placed.
Antibiotic prophylaxis in the perioperative period was performed in all cases with second-generation cephalosporins 30 minutes before surgery and during 5 days after.
During the first 6 weeks after the surgery, only partial weightbearing with 15 to 20 kg was allowed. Then weightbearing increased according to clinical and radiologic data acquired at followup visits scheduled at 6 to 8, 12 to 14, and 18 to 20 weeks after surgery and further with 6-week intervals if needed until fracture union.
For last followup, patients were recalled specifically, so terms of this examination differed from 12 to 48 months after surgery (mean, 23 months). At each visit we determined the ROM of adjacent joints and assessed axial and rotational malreduction. We noted when patients were allowed to bear full weight and resume work and sport activities. Function was graded according to Lower Extremity Functional Score (LEFS) [2] at 1-year followup. LEFS contains 20 questions about a person’s ability to perform everyday tasks like walking, squatting, and running with a total of 80 points possible. Any wound complications or other consequences were noted and graded according to classification of surgical complications of Dindo et al. [6]. According to this classification, Grade I complications needed simple opening of the wound at the bedside; Grade II, additional antibiotic treatment; Grade IIIA required closure of the wound under local anesthesia; and Grade IIIB needed general anesthesia and removal of hardware.
AP and lateral views were obtained at each visit. Both of us (AAS, the treating surgeon, and AVB, not the treating surgeon) evaluated the serial radiographs to assess healing. The fracture was considered united if three of four cortices showed bony bridging and full weightbearing was pain-free. If fracture union was not achieved by the sixth month after surgery, the situation was graded as delayed union and by the ninth month as nonunion. We assessed deformities in sagittal and frontal planes and shortening on standard long-leg radiographs. The joint orientation angles were used to access axial deviation in frontal and sagittal planes (medial proximal tibial angle normal range 87° ± 2.5°, lateral distal tibial angle 89° ± 3°, proximal posterior tibial angle 80° ± 3.5°, anterior distal tibial angle 79.8° ± 1.6°) [15]. We considered any malalignment in frontal and sagittal planes exceeding 5° from indicated limits as clinically important. The length was accessed both clinically and radiographically (as a distance between the centers of knees and ankles with respect to radiologic magnification). Rotational deformities were evaluated on CT scans available for 48 patients according to previously described methods [4, 18]. CT was performed as standard postoperative control during the last years of work. Rotational malalignment exceeding 10° was considered clinically important.
There were no missing data for these 80 patients. The arithmetic mean and SD were indexed through the text. Data were analyzed with the use of Microsoft Office Excel 2003 (Microsoft Inc, Redmond, WA, USA).
Results
Sixty-three of 80 patients (79%) had uneventful healing of the fractures. Mean time to full weightbearing was 5 ± 1.24 months (range, 1–13 months). Five of 80 fractures were considered radiographically healed at 14 weeks after surgery, 31 at 20 weeks, 34 at 6 months, and another three at 8 months. Delayed union was seen in three patients (4%). Nonunions were established in seven patients (9%). Thus, the overall rate of healing problems occurred in 10 patients (13%). In delayed unions, the consolidation of fractures and possibility of full weightbearing were achieved by 7 to 8 months without additional intervention. In nonunions, additional surgeries were performed (total of seven in this series): bone grafting in three, conversion to IMN in two, Ilizarov fixator in one, and plate and refixation with valgus correction in one. Five of the seven patients with nonunion had severe soft tissue injuries: Gustilo-Andersen III open fractures in three and Tscherne II closed injuries in two cases. In one patient, the reason for nonunion was apparently unrecognized intraoperative distraction of the fracture with valgus deformity of the distal tibia fragment (Fig. 1). Nonunion also occurred in the proximal third of the tibia in a 76-year-old woman with concomitant diabetes and spine fracture. The patient did not bear weight during the first 5 months after the injury. Conversion to IMN was performed 1 year after initial surgery.
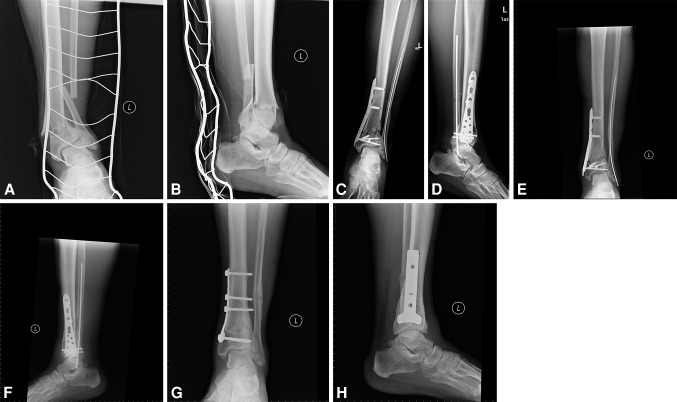
Fig. 1A–H.
A 46-year-old patient sustained a 43C1 fracture during a fall on the street (A–B). Skeletal traction for 8 days was used, then MIPPF of the tibia with a distal medial tibia locking plate was performed. The fibula was fixed with an intramedullary rod before tibia fixation. Postoperative radiographs revealed overdistraction at the fracture site and valgus malrepositioning (C–D). The patient refused further surgery until 6 months after injury. By this time, serial radiographs showed no positive signs of fracture healing (E–F). The locking plate was removed and fixation in the correct position with a 4.5-mm T-plate was performed. This led to healing of the fracture. Full weightbearing was resumed at 3 months after reoperation. Radiographs 1 year after reoperation are shown (G–H).
We identified wound complications in seven patients (8.75%). In all cases delayed (three) or late (four) infections occurred [20]. Superficial infection was seen in three cases and nonoperative treatment led to elimination of infection in them (Grade I). In another four patients deep infection occurred with skin breakdown up to the plate (screws). The hardware was removed early as soon as the fracture united (from 5 to 7 months); débridement at the time of secondary intervention led to elimination of infection (Grade IIIB).
Malalignment exceeding 5° in the frontal plane occurred in five (6.25%) patients (varus 7° and 9°; valgus 7°, 8°, 10°). One patient had an 8° valgus deformity of the distal tibia and nonunion; surgical treatment was performed 6 months after the injury: the locking plate was removed and varus osteotomy of the distal tibia with 4.5-mm T-plate fixation in correct position led to union (Fig. 1). We identified no patients with malalignment in the sagittal plane. CT evaluation of rotational deformities was performed in 48 cases. Internal rotation up to 10° was revealed in 19 patients, from 11° to 20° in six, and more than 20° in three patients. External rotation up to 10° occurred in 17 patients and 11° or more in three patients. Thus, a dedicated study of rotational deformities with the use of CT revealed that in 25% (12 from 48 examined patients), deviations greater than 10° were seen.
The mean LEFS score was 70 points from 80 possible at 1 year; restrictions arose from difficulties with running and hopping. Sixty-seven of 80 patients completely resumed work activities. Twenty-two of 27 patients who were engaged in recreational sports resumed their activities. Restriction of motion in the ankle exceeding 5° occurred in seven patients with distal intraarticular tibia fractures. In one patient with a proximal tibia fracture combined with a distal femoral fracture in the same leg, the restriction of knee extension required arthroscopic arthroplasty with residual 5° deficit of extension at the last followup 18 months after the injury.
Discussion
MIPPF is gaining wide application in the treatment of periarticular fractures of the tibia [3, 5, 7, 9, 12, 14, 16, 17]. Despite wide acceptance and assurance in possibilities of the procedure, most reports are based on a small number of patients and the investigators report differing rates of wound complications, time to union, malalignment, and function (Table 2), thus posing questions about whether the theoretical advantages are achieved. Thus, our aims were to estimate the rate of union in tibial fractures treated by MIPPF, the rate of major and minor wound complications, occurrence of malunion, and level of function.
Table 2.
Results of MIPPF in tibia fractures
| Publication | Number of patients reported/available for followup | Consolidation disturbances | Axial deviations (> 5°) | Wound problems/infection | Revision surgeries |
|---|---|---|---|---|---|
| Borg et al., 2004 [3] | 21/21 | 4/21 (19%) | 6 (28.5%) (2 reoperations) | 3 (14.3%) | 4 (19%) |
| Mafulli et al., 2004 [12] | 20/19 | 1/19 (5.3%) | 7/19 (36.8%) | 0 | 2 (10.5%) |
| Redfern et al., 2004 [16] | 20/20 | 0/20 | 1/20 (5%) | 1/20 (5%) | 1 (5%) |
| Hasenboehler et al., 2007 [9] | 32/29 | 5/29 (17.2%) | 0 | 1/29 (3.4%) | 1/29 (3.4%) |
| Williams and Schenk, 2008 [21] | 20/19 | 6/19 (31.5%) | NA | 2/19 (10.5%) | 1 (5.3%) |
| Lau et al., 2008 [11] | 48 | 5/48 (10.4%) | NA | 8/48 (16.7%) | 4 (8.3%) |
| Gupta et al., 2010 [7] | 79/71 of them MIPPF | 10/79 (12.7%) | 2/79 (2.5%) | 3/79 (3.8%) | 5* (6.3%) |
| Ronga et al., 2010 [17] | 21 | 1 (4.8%) | 4 (19%)† | 9 (42.3%) | 0 |
| Current study | 80 | 10/80 (13%) | 5/80 (6%) | 7/80 (9%) | 7 (9%) |
* Beside this, five patients required primarily bone grafting; †no one more than 7°; MIPPF = minimally invasive percutaneous plate fixation; NA = data not available.
The limitations of the study include the following. First, our cohort involves both proximal and distal fractures of the tibia and fractures with various degrees of soft tissue injuries, which makes our group not completely homogenous. Second, a large portion of the patients (16 cases) was examined at 2 or more years after the injury, and patient answers about terms of restoring weightbearing or resumption of working capacity would be subject to recall bias and may be not accurate. Third, function in some patients could be studied at 24 to 48 months after surgery, whereas in other patients, the last examination was performed 1 year after surgery. Because function might improve after 1 year, our findings might underestimate mean function. Fourth, all the patients were evaluated by a single individual (AS) who performed most of the surgical cases in this series raising a question of bias in recording key parameters. We did have followup of 80 of 89 patients treated with MIPPF. Unlike other investigations with more strict inclusion criteria (only distal tibia fractures [7, 16] or fractures treated by single surgeon [17]), all patients treated with MIPPF were included without regard for fracture location (upper or lower third, shaft), degree of soft tissue injury, terms of surgery, or experience of the treating surgeon. We believe this will allow better understanding of real possibilities of the method on the whole.
Rates of fracture union vary in the literature from 68.5% [21] to 100% [16] (Table 2). Most authors report union in terms of 4 to 6 months after MIPPF. Authors from Asia typically report shorter times to union, 4 to 4.5 months [7, 10], than authors from Europe, 5 to 6 months [3, 9, 16]. In our series, fracture union was seen in 70 (88%) cases up to 6 months after surgery. The incidence of consolidation disturbances also depends on the severity of original trauma. Collinge et al. [5] report an approximately 42.8% rate of delayed union, but 12 of the 17 patients had open fractures and 11 had polytrauma. Most current articles report about a lower level of these complications [3, 5, 7, 9, 12]. In our group, 10 patients had delayed union or nonunion (12.5%). Seven of these 10 patients had severe injuries of soft tissues (Gustilo III open fractures in four, Tscherne II closed fractures in three). When only fractures without major soft tissue injuries are taken into account, complicated union was seen only in three of 67 patients (4.5%).
Minimization of the skin incision and further soft tissue injury within the fracture site should decrease the rate of wound complications. In our series, such complications were seen in seven patients (9%), which is within the range reported in most studies. The rate of wound complications obviously depends on severity of initial trauma and degree of soft tissue injury, and in three of these patients initially, severe injuries of soft tissues (Gustilo II–III open fractures) were seen. All wound infections in our series were delayed or late infections, which is consistent with data of Lau et al. [11], who revealed late infections in 15% of their cases. Like in the series of Lau et al., infection complications that were seen in our cases did not affect fracture healing and did not lead to chronic osteomyelitis.
Axial malalignment poses another potential issue in MIPPF because of potential difficulties of intraoperative control of fracture reduction. Some studies report a relatively low level of axial malalignment from 0% to 5% [7, 9, 16], whereas others state up to 28% to 37% of axial deviations exceeding 5° [3, 12]. In our study, deformities from 5º to 10° were seen in five (6.25%) patients. Two of these patients were operated on relatively late (24 and 45 days after the injury), which could make the reduction more difficult. Rotational malalignment is difficult to assess clinically and there are just a few articles that address this issue [4, 18]. We performed CT to evaluate rotational malalignment in 48 consecutive patients when CT became available for routine investigations. Deviations exceeding 10° were revealed in 12 patients (25% of examined patients). It must be pointed out that calculation of rotational deformity was performed in comparison to healthy contralateral tibia. Strecker et al. [18] described normal differences in tibia torsion up to 14°. Thus, the clinical importance of the rotational malalignment we observed is unclear and needs further investigation.
Function after MIPPF of tibia fractures is not the focus of attention in most studies and function is difficult to compare because of different locations of fractures (intra- or extraarticular or shaft fractures) and different methods used for evaluation. Most cited results are ROM in the adjacent joint and resumption of work and sport activity at followup. Thus, Redfern et al. [16] reported that all their 20 patients had resumed their preinjury level of activity. On the contrary, Borg et al. [3] reported that only 10 of 21 patients had full ankle ROM after a distal tibia fracture and that 11 of 21 patients did not resume their preinjury sporting and leisure activities at latest followup. Ronga et al. [17] revealed that 16 of their 19 patients had not returned to their preinjury activities and five patients had limitation in the ROM more than 20° compared with the contralateral side. We noticed restrictions of ROM in eight of our patients. Functional results were studied according to LEFS [2]. The mean result comprised 70 points (from a maximum of 80). Sixty-seven of 80 of our patients (84%) had completely resumed work activities as well as 22 of 27 patients who were engaged in recreational sports (81%).
MIPPF allowed uneventful healing in 79% of our cases. Consistent with the literature, a high percentage of our patients has resumed their preinjury level of sports and working activities with general restoration of lower leg function. However, complications occurred in a substantial portion of patients, which may be divided into three groups: disturbances of fracture healing (13%), wound complications (9%), and axial malalignment (6%). The limited number of patients with severe soft tissue injuries and late MIPP fixation does not allow us to make definitive conclusions, but it is our belief that in this subgroup of patients, MIPPF should be used with caution because of the higher potential risk of mentioned complications. The causes of complications need further investigations. In patients without substantial soft tissue injury, the complication rate was low.
Acknowledgments
We thank N. Zhizhko-Mikhasevich, MD, and I. Somova, MD, for assisting in calculation of rotational deformities.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Babst R, Khong K. Minimally invasive surgery. In: Rüedi TP, Buckley RE, Moran CG, eds. AO Principles of Fracture Management. Stuttgart, Germany; New York, NY, USA: Thieme; 2007:199–212.
- 2.Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application, North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79:371–383. [PubMed] [Google Scholar]
- 3.Borg T, Larsson S, Lindsjo U. Percutaneous plating of distal tibia fractures. Preliminary results in 21 patients. Injury. 2004;35:608–614. doi: 10.1016/j.injury.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Buckley R, Mohanty K, Malish D. Lower limb malrotation following MIPO technique of distal femur and proximal tibial fractures. Injury. 2011;42:194–199. doi: 10.1016/j.injury.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Collinge C, Sanders R, DiPasquale T. Treatment of complex tibial periarticular fractures using percutaneous technique. Clin Orthop Relat Res. 2000;375:69–77. doi: 10.1097/00003086-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Dindo D, Demartines N, Clavien P. Classification of surgical complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta RK, Rohilla RK, Sangwan K, Singh V, Walia S. Locking plate fixation in distal metaphyseal tibial fractures: series of 79 patients. Int Orthop. 2010;34:1285–1290. doi: 10.1007/s00264-009-0880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 9.Hasenboehler E, Rikli D, Babst R. Locking compression plate with minimally invasive plate osteosynthesis in diaphyseal and distal tibial fracture: a retrospective study of 32 patients. Injury. 2007;38:365–370. doi: 10.1016/j.injury.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. doi: 10.1086/646436. [DOI] [PubMed] [Google Scholar]
- 11.Lau TW, Leung F, Chan CF, Chow SP. Wound complication of minimally invasive plate osteosynthesis in distal tibia fractures. Int Orthop. 2008;32:697–703. doi: 10.1007/s00264-007-0384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maffulli N, Toms A, McMurtie A, Oliva F. Percutaneous plating of distal tibial fractures. Int Orthop. 2004;28:159–162. doi: 10.1007/s00264-004-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller ME, Nazarian S, Koch P, Schatzker J, Heim U. The Comprehensive Classification of Fractures of Long Bones. Berlin, Heidelberg, Germany; New York, NY, USA: Springer-Verlag; 1990. [Google Scholar]
- 14.Nikolaou V, Efstathopoulos N, Papakostidis C, Kanakaris N, Giannoudis P. Minimally invasive plate osteosynthesis—an update. Curr Orthop. 2008;22:202–207. doi: 10.1016/j.cuor.2008.04.004. [DOI] [Google Scholar]
- 15.Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25:425–465. [PubMed] [Google Scholar]
- 16.Redfern DJ, Syed SU, Davies SJM. Fractures of the distal tibia: minimal invasive plate osteosynthesis. Injury. 2004;35:615–620. doi: 10.1016/j.injury.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Ronga M, Longo UG, Mafilli N. Minimally invasive locked plating of distal tibia fractures is safe and effective. Clin Orthop Relat Res. 2010;468:975–982. doi: 10.1007/s11999-009-0991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strecker W, Popp D, Keppler P. Torsional deformities following intramedullary nailing of femur and tibia. Osteo Trauma Care. 2004;12:215–218. doi: 10.1055/s-2004-832297. [DOI] [Google Scholar]
- 19.Tscherne H, Gotzen L. Fractures With Soft Tissue Injuries. Berlin, Heidelberg, Germany; New York, NY, USA: Springer-Verlag; 1984. [Google Scholar]
- 20.Willenegger H, Roth B. Treatment tactics and late results in early infection following osteosynthesis] [in German. Unfallchirurgie. 1986;12:241–246. doi: 10.1007/BF02586085. [DOI] [PubMed] [Google Scholar]
- 21.Williams T, Schenk W. Bridging-minimally invasive locking plate osteosynthesis (Bridging-MILPO): technique description with prospective series of 20 tibial fractures. Injury. 2008;39:1198–1203. doi: 10.1016/j.injury.2008.05.008. [DOI] [PubMed] [Google Scholar]