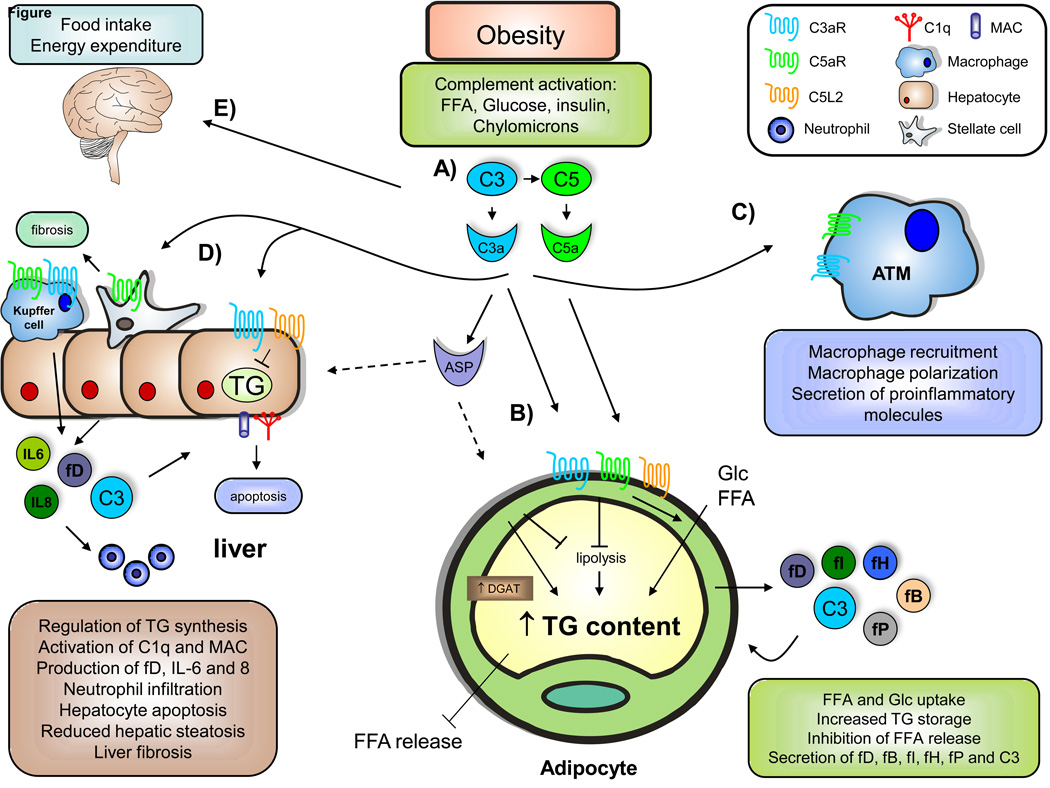
Fig. 1. The role of complement in obesity-related metabolic disorders.
A) During obesity, increased plasma and tissue concentrations of free fatty acids (FFA), glucose (Glc), insulin, and chylomicrons are found. These factors can promote complement activation, resulting in the production of elevated levels of anaphylotoxins C3a and C5a as well as C3adesArg (ASP), both locally and systemically. B) By the binding of C3a and C5a to their respective receptors C3aR and C5aR, complement components could promote triglyceride (TG) formation through the inhibition of lipolysis, enhanced Glc and FFA uptake, and indirect reduction in FFA release. In addition, C5L2 has been shown to promote lipid incorporation into TG via diglyceride acyltransferase (DGAT) activation. Adipocytes in the obese AT are able to produce several complement factors, including factor D (adipsin), fP (properdin), factor B, factor H, and C3. C) Anaphylotoxin C3a, and potentially C5a, can promote adipose tissue macrophage (ATM) recruitment, macrophage polarization to a pro-inflammatory phenotype, and secretion of pro-inflammatory factors, contributing to obesityinduced insulin resistance. D) C3a can potentially regulate TG accumulation in hepatocytes and hepatic steatosis. C3 has also been linked to C1q and membrane attack complex (MAC) deposition as well as hepatocyte apoptosis, although the exact mechanisms remain unclear. In addition, hepatocytes can promote neutrophil recruitment via pro-inflammatory factors such as IL-6 and IL-8, as well as complement activation. Furthermore, C5a has been implicated in liver fibrosis development. E) Complement has also been shown to impair food intake and energy expenditure by acting on the central nervous system.