Table 1.
Solvent and additive effect in the selective formation of triene 4 a or vinylallene 5 a.[a]
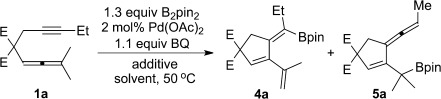 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Additive (20 mol %) | Time [h] | Yield of4 a/5 a [%][b] | 4 a/5 a |
| 1 | THF | – | 15 | 28:14 | 2:1 |
| 2 | cyclohexane | – | 15 | 33:34 | 1:1 |
| 3 | CH2Cl2 | – | 15 | 39:35 | 1:1 |
| 4 | DCE | – | 15 | 61:13 | 5:1 |
| 5 | DCE | Na2CO3 | 15 | 70:7 | 10:1 |
| 6 | DCE | NaOAc | 15 | 67:6 | 11:1 |
| 7 | DCE | LiOAc⋅2H2O | 15 | 73:7 | 10:1 |
| 8[c] | DCE | LiOAc⋅2H2O | 15 | 71:10 | 7:1 |
| 9 | THF | HOAc | 20 | 19:16 | ca. 1:1 |
| 10 | THF | p-TSA | 20 | 0 | – |
| 11 | THF | BF3⋅Et2O | 20 | 3:78 | 1:26 |
| 12[d] | THF | – | 20 | 5:60 | 1:12 |
| 13 | THF | Et3B | 20 | 8:24 | 1:3 |
Unless otherwise noted, 1 a, B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), and indicated additive (20 mol %) were dissolved in the indicated solvent (5 mL mmol−1) and stirred at 50 °C in a sealed tube.
Yield was determined by 1H NMR spectroscopy using anisole as internal standard.
50 mol % of LiOAc⋅2H2O was added.
2 mol % of [Pd(CH3CN)4][(BF4)2] was used in place of Pd(OAc)2. E=CO2Me.
