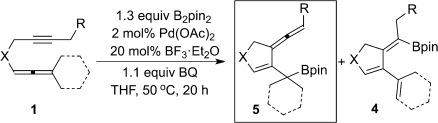
Table 3.
Selective carbocyclization of allenynes 1 yielding borylated vinylallenes 5[a]
 | ||||
|---|---|---|---|---|
| Entry | Allenyne | Product | 5/4[b] | Yield of 5 [%][c] |
| 1 | 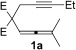 |
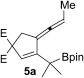 |
>20:1 | 77 |
| 2 | 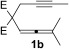 |
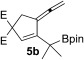 |
>20:1 | 73 |
| 3 | 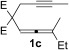 |
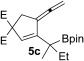 |
>20:1 | 56 |
| 4 | 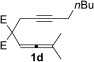 |
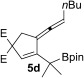 |
>20:1 | 79 87[d] |
| 5 | 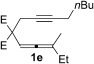 |
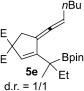 |
20:1 | 77 |
| 6 | 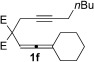 |
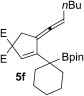 |
>20:1 | 70 |
| 7[e] | 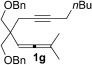 |
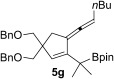 |
>20:1 | 37 |
Unless otherwise noted, 1 (0.1–0.2 mmol), B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), and BF3⋅Et2O (20 mol %) were dissolved in THF (5 mL mmol−1) and stirred at 50 °C for 20 h.
Ratio determined by 1H NMR analysis of the crude reaction mixture.
Yield of the isolated product.
1 mmol of 1 d was used.
2 mol % of [Pd(CH3CN)4][(BF4)2] was used in place of Pd(OAc)2 and BF3⋅Et2O. E=CO2Me.
