Abstract
Background & objectives:
HIV/AIDS patients may have renal involvement also, however, Indian data are sparse. The present study was done to find the spectrum of renal diseases in HIV/AIDS patients in north India.
Methods:
In this prospective pilot study, HIV positive patients aged >18 yr were screened for renal involvement [serum creatinine >1.5 mg% and/or significant proteinuria (>500 mg /day)]. Patients who were positive on screening were followed up prospectively and underwent kidney biopsy if indicated.
Results:
A total of 526 patients were screened, of these, 91 (17.3%) were found to have renal involvement. Group A (Treatment naïve) comprised 392 patients who were not on antiretroviral treatment (ART) and group B (patients on ART) comprised 134 patients. More patients (74/392, 18.9%) in group A had renal involvement as compared to patients in group B (17/134, 12.7%). Of the 91 patients with renal involvement, 26 were followed up and underwent kidney biopsy. Thirteen patients had only proteinuria and another 13 had renal dysfunction with or without proteinuria. Most common histological diagnosis was mesangioproliferative glomerulonephritis (mes PGN) (10/26). Two patients had collapsing FSGS (focal segmental glomerulosclerosis) and three patients had immune complex glomerulonephritis. Seven patients had acute kidney injury, whom six totally recovered from their renal function. All patients with mesPGN tolerated angiotensin converting enzyme (ACE) inhibitors well. There was mixed response of collapsing FSGS to steroids. Both patients with MPGN (membranoproliferative glomerulonephritis) did well on low dose of steroid and ART.
Interpretation & conclusions:
Renal involvement was found to be common in HIV positive patients (17.3%). A low occurrence of renal involvement found in patients already on ART suggests some renoprotective effect of ART. Our preliminary results showed that collapsing FSGS was not rare in Indian HIV positive population, but classical HIV associated nephropathy was not seen. Longitudinal studies with robust study design and large sample size need to be done to confirm the findings.
Keywords: ART, human immunodeficiency virus (HIV), nephropathy, renal disease
About 34.2 million people worldwide have been infected with the human immunodeficiency virus (HIV)1. Approximately 2.3 million people were estimated to be infected with HIV in India in 2009 and the estimated adult prevalence was 0.31per cent2. HIV infection affects multiple organs and kidney is a common target. A variety of renal syndromes may occur during the course of HIV infection3. These can be either acute or chronic. Acute renal failure (ARF) has been reported in up to 10 per cent HIV infected patients attending outpatient HIV clinic4. More than one-half of these episodes were attributed to underlying infections; drug-related complications accounted for nearly one-third of cases and liver disease accounted for 10 per cent of cases4.
HIV associated nephropathy (HIVAN) is one of the chronic kidney diseases directly caused by HIV infection5. In early 1980s, it became apparent that HIV might also be associated with a more specific form of glomerular disease. Reports from Miami and New York described a characteristic renal lesion named HIV-associated nephropathy (HIVAN), with histological features of focal and segmental glomerulosclerosis which was associated with nephrotic range proteinuria and rapidly progressive renal failure6,7,8. Majority of these cases were found among blacks (Afro Americans) heterosexual i.v. drug abusers. The study by Bourgoignie9 represented the same in 83 per cent of the histological renal specimen. However, HIVAN was rare among white American homosexuals10,11,12. Studies from Europe13,14 have also found a low incidence of HIVAN in Caucasian and Asian patients as compared to blacks. Naaz et al15 from India reported first case of classical HIVAN from the State of Jammu and Kashmir, a low incident belt for HIV. Subsequently another study from India reported two patients of HIVAN presented with nephritic range proteinuria with renal involvement in HIV infected children16.
HIV infection may also be associated with other forms of renal diseases such as IgA nephropathy, immune complex nephritis and interstitial nephritis13,17. A cross-sectional study from South Africa revealed HIVAN in 83 per cent, membranoproliferative nephropathy in 7 per cent and interstitial nephritis in 10 per cent of patients18. Eventually three syndromes have been recognized to cause chronic nephropathy in HIV/AIDS patients, namely, classic HIVAN, HIV associated immune complex diseases and thrombotic microangiopathies [HIV-associated thrombotic thrombocytopenic purpura (TTP)/haemolytic uremic syndrome (HUS)].
There is paucity of data from India regarding renal involvement in HIV disease. Hence this pilot study was done to find the spectrum, clinical course and impact of treatment on renal diseases in Indian HIV/AIDS patients.
Material & Methods
This is a single centre prospective observational study done from April 2006 to November 2007 at the All India Institute of Medical Sciences (AIIMS) hospital, New Delhi, a tertiary care center of north India. Ethical approval for the study protocol was obtained from Ethics Committee of AIIMS. All consecutive HIV seropositive patients attending the outpatient HIV clinic, Renal Clinic or admitted under Medicine or Nephrology services, were considered for screening. Written informed consent was obtained from all patients. Being a pilot study, initially sample size calculation was not done and subjects were taken as a sample of convenience.
All HIV positive patients were screened for spot proteinuria (using standard urine dipstick testing) and serum creatinine. Patients aged ≥18 yr were included with serum creatinine ≥1.5mg% and/or they had ≥1+ spot proteinuria. Patients with pre-existing chronic renal disease not related to HIV (e.g. renal diseases due to diabetes mellitus, hypertension and collagen vascular diseases etc.) or having confounding factors for proteinuria (such as heavy exercise, cardiac failure, hyperglycaemia, uncontrolled hypertension, acute illness and urinary tract infection) were excluded from the study. The included patients underwent a 24-h urinary protein and creatinine estimation. A repeat serum creatinine was done and spot urine sample were also taken. Patients with serum creatinine ≥1.5mg% were labelled as having renal dysfunction. Patients with renal dysfunction and/or spot proteinuria > 1+ or 24-h urinary protein ≥500mg were classified as having renal involvement. Nephrotic range proteinuria was defined as a 24-h urine protein ≥3.5. Creatinine clearance was calculated from 24 h urine creatinine estimation which was done along with 24 h urinary protein.
All screened patients were divided into two groups. HAART (highly active anti retroviral therapy) naïve cases were categorized in Group A and patients taking HAART were classified as Group B. HAART consisted of two of three nucleoside reverse transcriptase inhibitors (NRTIs) (zidovudine, lamivudine and stavudine) and one of the two non-nucleoside reverse transcriptase inhibitors (NNRTIs) (nevirapine or efavirenz). Drugs were supplied free of cost under the National AIDS Control Programme (National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India). No protease inhibitors were supplied.
A detailed history, clinical examination and laboratory investigations were carried out for all enrolled patients. Information was specifically collected pertaining to age and gender, risk factor/s for HIV infection, presence of any opportunistic infections, blood pressure, duration of hypertension (if present), renal function deterioration and duration of renal failure (if available), complete blood count, urinalysis, blood urea, CD4/CD8 counts (BD FACS Count system, USA), HBsAg (Hepanostika® HBsAg Ultra; bioMérieux, France), Anti-HCV antibodies (EasyQ HCV, bioMérieux, France) and C3 and C4. Data were also collected pertaining to ultrasonography of kidneys, ureters and urinary bladder, duration and type of HAART. Renal biopsy was done, after obtaining written informed consent, to know the histological diagnosis. All renal biopsies were examined by a single nephropathologist who had prior information about the patients’ HIV status and clinical background. Immunofluorescence microscopy was not done on kidney biopsies as per institute's policy. However, immunohistochemistry (IHC) was done on 5 micron thick paraffin sections cut on poly-L-lysine coated slides. The sections were trypsinized for antigen retrieval. IHC was performed using Avidin-Biotin-Conjugate (ABC) kit (Vector Lab, USA) with antibody against IgA, IgG, IgM, C3 and fibrin (Sigma Chemicals, USA).
Patients were regularly followed up every month during the study period on mortality and cause of death was collected.
Statistical analysis: The patients were divided into group A (treatment naïve) and group B (patient on HAART). The patients in each group were further divided into patients with and without renal involvement (PWRI). The latter group was referred to as “PWRI group”. To analyze the association between categorical and continuous variables, two-sample t-test and Chi-square test were used, respectively. P<0.05 was taken as significant. All analysis was done by Graph Pad version 5 (USA).
Results
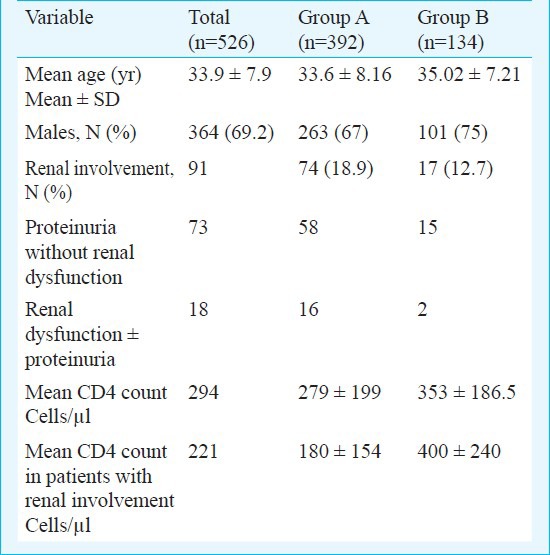
A total of 526 HIV positive patients were screened during the study period. The baseline characteristics of total patients are shown in Table I. Of the 526 patients, 91 (17.3%) were found to have renal involvement (proteinuria or renal dysfunction). The mean serum creatinine was 0.85 ± 0.16 mg% in proteinuric group with 86% (63/73) of the patients had ≥3+ proteinuria. Patients with renal dysfunction had a mean serum creatinine of 4.60 ± 3.17 mg% (range 1.7-10.5). The mean CD4 count of Group A was significantly lower in patients with renal involvement as compared to PWRI group (180 ± 154 vs. 302 ± 201 cell/μl, P<0.0001) while this was not so in Group B (400 ± 240 vs. 346 ± 178 cell/μl).
Table I.
Descriptive characteristics of the study group and the two groups based on HAART
Of the 91 patients with renal involvement on initial screening, only 26 came for follow up. They got repeat spot urine examination, repeat serum creatinine and 24 h urine protein and creatinine and underwent renal biopsy. Of the remaining 65 patients, 25 were referred to ART clinics of other government hospitals for further follow up (as per policy of our ART clinic), 34 were lost to follow up and the remaining six patients did not give consent for further study.
Out of 26 patients on follow up, 21 were males. Mean age was 36.5 yr with a range of 25-45 yr. The presenting features (multiple in many) were fever in nine (35%), oedema in seven (27%), oliguria in five (19%), weight loss in four (15%), gastroenteritis in two (7%), and altered sensorium in one (4%) patient. Seven (27%) patients were asymptomatic. The commonest mode of transmission was through heterosexual exposure in 20, in three it was through injections/blood products, while it was unknown in the remaining three. Thirteen patients (50%) had only proteinuria and 13 had renal dysfunction (with or without proteinuria). Hypertension was present in five patients. Mean duration of HIV infection was 12.8 months with a range of 1-84 months. Mean follow up of patients was eight months with a range of 4-20 months.
Histopathological results: The light microscopy findings of the 26 patients have been described in Table II and Figs 1, 2 and 4. Kidney biopsy samples were processed for electron microscopy (EM) in 17 cases although adequate sample (containing glomeruli) was present only in nine cases. EM findings of a representative biopsy specimen is shown in Fig. 3.
Table II.
Light microscopy (LM) findings of the study group (n=26)
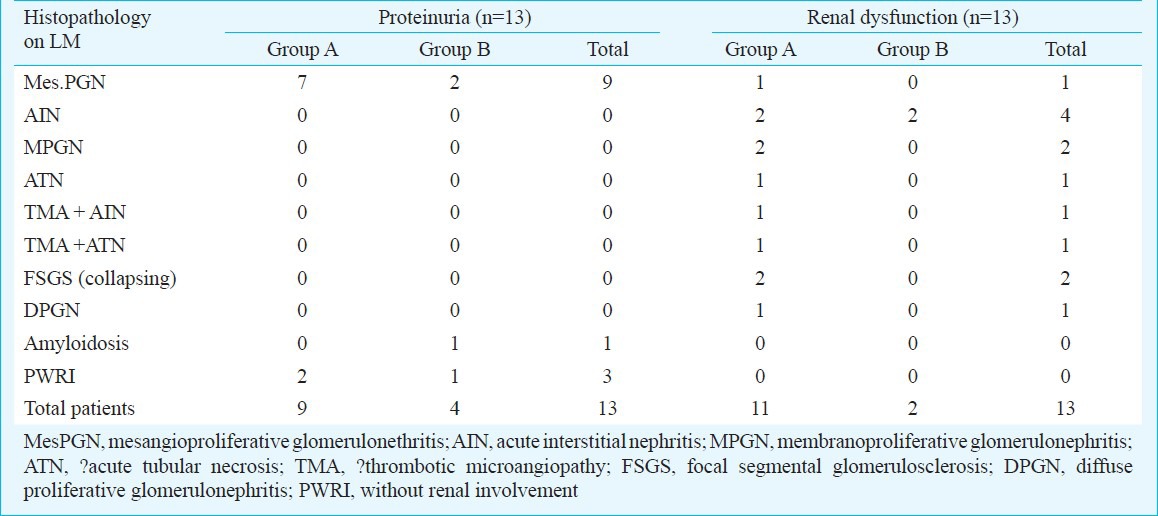
Fig. 1.
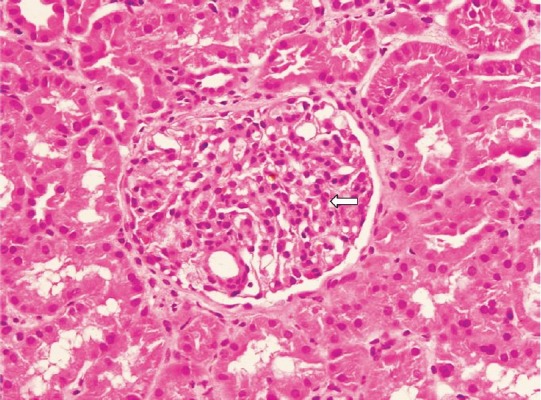
Light microscopic photomicrograph of kidney biopsy sample of a patient with renal involvement showing glomeruli with mesangial cell proliferations compatible with mesangioproliferative glomerulonephritis (H & E stain, 400X). Arrow shows glomeruli.
Fig. 2.
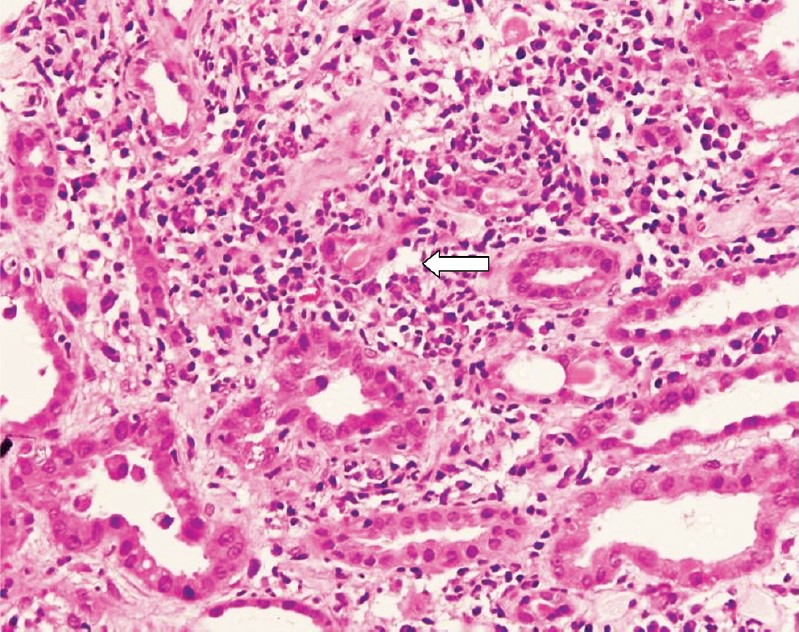
Light microscopic photomicrograph of kidney biopsy sample of a patient with renal involvement showing acute and chronic interstitial inflammation (white arrow) with destruction of tubular epithelial cell and tubulitis. Features are compatible with acute interstitial nephritis (H & E stain, 400X).
Fig. 3.
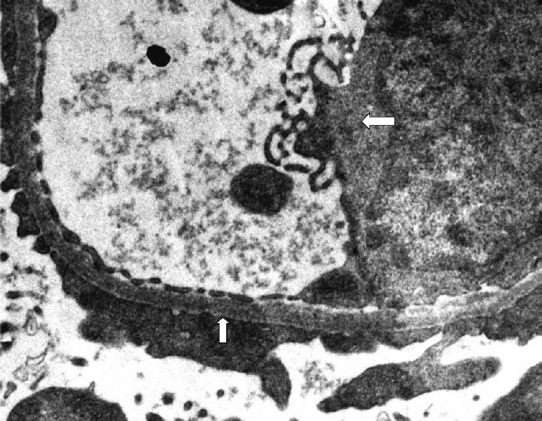
Electron microscopic photograph of kidney biopsy sample of patient with renal involvement showing endothelial cell swelling left point (white arrow) with degenerative changes and it also shows effacement of foot process upward pointing (white arrow) indicative of the podocyte injury/podocytopathy (40,000X).
Patients in proteinuria group (n=130) had mean 24 h proteinuria of 1561 ± 906 mg with a range of 600- 3900 mg/day. Only one patient had nephrotic range proteinuria. Mean estimated creatinine clearance (by 24 h urinary creatinine measurement) was 69 ± 12 ml/min. Nine patients in this group had mesangioproliferative glomerulonephritis (Mes PGN) on light microscopy. Eight of these biopsies were also examined by electron microscopy. Three patients did not have adequate sample to comment on glomeruli but one of them showed viral particles in tubular epithelial cell. Of the remaining five, two showed additional findings of segmental sclerosis with focal foot process effacement labelled as evolving secondary focal segmental glomerulosclerosis (FSGS) and other two showed only focal foot process effacement labelled as having podocytopathic changes. One patient had amyloidosis on light microscopy. Three biopsies were reported normal on light microscopy. All of them had 24 h proteinuria between 500-1000 mg. Only one of these three biopsies had glomeruli on electron microscopy, which had focal foot process effacement labelled as having podocytopathic changes.
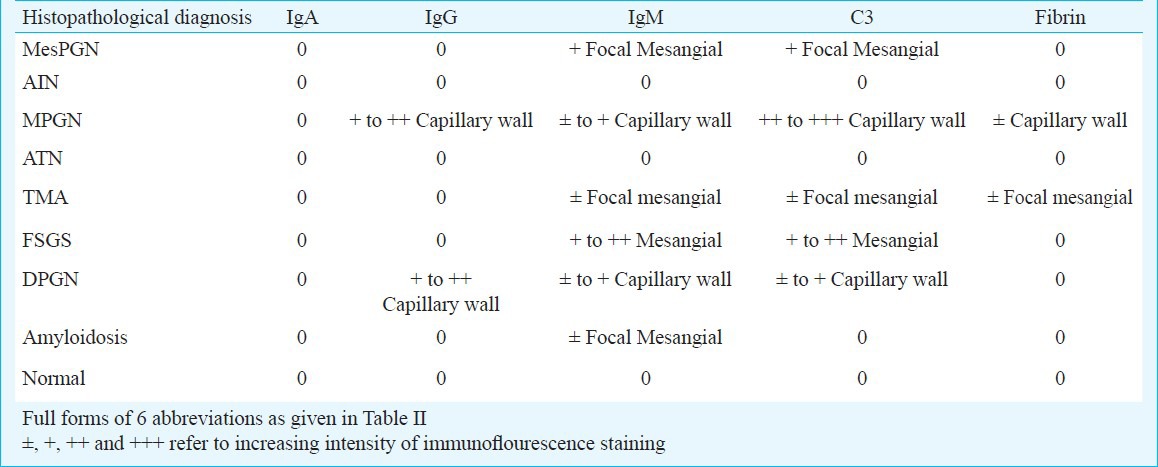
There were 13 patients in renal dysfunction group having mean estimated creatinine clearance of 14 ± 11 ml/min. Of these, seven patients had only renal dysfunction and six had concurrent proteinuria also. Of these seven patients, six had totally recovered renal function, four had acute interstitial nephritis (AIN, drug induced), one had acute tubular necrosis (ATN, post gastroenteritis volume depletion), and one patient had thrombotic microangiopathies (TMA) with AIN. One of the AIN patients revealed segmental sclerosis with focal foot process effacement (evolving secondary FSGS) on EM. One patient had TMA with ATN who progressed to end stage renal disease requiring dialysis and later lost to follow up. Six patients had renal dysfunction with proteinuria. Of these, three patients had immune complex diseases [two had membranoproliferative glomerulonephritis (MPGN) and one had diffuse proliferative glomerulonephritis (DPGN) (lupus like, got IF done outside our hospital which showed granular glomerular staining for IgG, IgA, IgM, C3 and C1q and serum was negative for antinuclear antibodies (ANA) and anti double-stranded DNA, later on IHC was done at AIIMS which showed deposition of IgG, IgM and C3 in capillary wall as shown in Table III. Two patients had collapsing FSGS and one had mesPGN. None of the collapsing FSGS patients had microcystic tubular dilatation and interstitial cell infiltrate and hence could not be classified as classical HIVAN. Immunohistochemistry (IHC) was done in 19 patients and results are given in Table III.
Table III.
Findings of immunostaining in 19 patients with reference to light microscopy
Treatment and follow up: All patients with mesPGN (n=10) were treated with ACE (angiotensin converting enzyme) inhibitors (ramipri 2.5-10 mg/day). They maintained their renal function and one patient showed reduction in proteinuria (from 1200 to 500 mg/day). Their mean duration of follow up was 8 months (range 4 to 11 months).
Both patients with MPGN did well on low dose steroid (0.5 mg/kg/day) and ART (till last follow up of 1 year) with significant improvement in proteinuria and renal dysfunction.
The response of collapsing FSGS to steroids was largely mixed. One patient with moderate renal dysfunction (serum creatinine- 2.2mg%, creatinine clearance of 20.5 ml/min) and 24-h proteinuria of 1 g did well on ART and low dose steroid (0.5 mg/kg/day). He had creatinine clearance of 35 ml/min and proteinuria of 750 mg/day after 6 months of treatment. But the patient who presented with advanced azotemia (dialysis requiring) and 16 g/day of proteinuria did well initially on steroid (1 mg/kg/day) and ART and renal function recovered (became dialysis independent with creatinine clearance of 60ml/min and decline in proteinuria to 1.5 g/day) after 1 ½ months of treatment but later he died because of pneumonia and sepsis.
One patient with DPGN did not improve on steroid (1 mg/kg/day) and ART. This creative level gradually progressed and he succumbed to pneumonia and sepsis within 2 wk of start of treatment.
Discussion
This study examined HIV-infected subjects, predominantly in an ambulatory setting where majority (98.6%) were outpatients. Renal biopsies were performed in 26 patients with variable degrees of proteinuria and renal dysfunction, and 89 per cent biopsies showed positive histopathological finding indicating importance of screening tests for renal lesion in HIV patients.
Overt proteinuria has been encountered in 14-50 per cent of HIV/AIDS patients in various studies depending upon method of screening and patient population16,19,20,21,22. Crowley et al evaluating spot urine samples, reported prevalence of ≥1+ proteinuria in 22.4 per cent patients with prevalence of persistent proteinuria as 14 per cent21. Han et al18 did 24-h proteinuria quantification and reported prevalence of 6 per cent overt proteinuria and 8 per cent persistent microalbuminuria in treatment naïve HIV patients in South Africa. In our study, higher percentage of patients (80.21%) was found to have proteinuria.
In the present study 28.57 per cent patients were enrolled for renal biopsy and follow up, which was better than previous studies18,23. The significant drop out of patients underscores the social stigma of HIV, poor knowledge about HIV and overall illiteracy among our patients. Intravenous (i.v.) drug abuse was found to be a common mode of transmission of HIV by Praditpornsilpa et al24 from Thailand, but we did not find any such case in our study.
HIVAN has been found to be the commonest form of renal involvement in HIV-positive Black patients13,14,25. A report comparing the experience in European and North American centers26 showed that in Black patients with HIV, FSGS was the commonest lesion occurring in about 80 per cent of renal biopsies. However, studies from Italy27 and Thailand24 did not report any case with HIVAN. Varma et al22 from India described one patient with collapsing FSGS and four with non-collapsing FSGS out of total of 25 HIV positive patients. Another study from India reported first case of classical HIVAN from the State of Jammu and Kashmir, a low incident belt for HIV15. We found two cases showing collapsing FSGS on light microscopy and an additional EM finding of segmental sclerosis with focal foot process effacement (labelled as evolving Secondary FSGS) in three other biopsies. Classical HIVAN features of microcystic tubular dilatation and interstitial cell infiltrate were not seen. Our data indicate that collapsing FSGS may not be rare in this part of the world as was previously thought, but classical HIVAN is rare.
There were several limitations in our study. The study design involved a sample of convenience rather than a random sample. The definition of ‘renal involvement’ was based on the single value of proteinuria and/or serum creatinine, many patients were lost to follow up after initial screening, electron microscopy and immunohistochemistry could not be carried out on all samples, which might have detected more cases of immune complex diseases. Design of the study was prospective observational, rather than interventional one, and the study period was too short to identify the effect of HAART on renal involvement.
In conclusion, renal involvement was seen to be common in Indian patients with HIV. Proteinuria and elevated serum creatinine could be an early marker of HIV associated renal lesions and screening for their presence may be beneficial. Renal biopsy is considered beneficial in seropositive patients with proteinuria especially with low CD4 count for early diagnosis and treatment of renal lesion. A low occurrence of renal involvement found in patients already on ART suggests some renoprotective effect of ART. Collapsing FSGS was not found to be rare in Indian HIV positive patients, but classical HIVAN was not seen. The present findings need to be confirmed in further studies with larger sample size and longer duration of follow up.
Acknowledgment
Authors acknowledge the help of staff of ART Clinic, AIIMS, New Delhi, specially Dr Sanjai Ranjan and all the staff of Nephrology Department, AIIMS.
References
- 1.UNAIDS/WHO. AIDS epidemic update: November 2011. [accessed on May 11, 2012]. Available from: http://www.unaids.org .
- 2.UNAIDS/WHO. UNAIDS report on the global AIDS epidemic 2010. [accessed on May 11, 2011]. Available from: http://www.unaids.org .
- 3.Weiner NJ, Goodman JW, Kimmel Pl. The HIV-associated renal diseases: current insight into pathogenesis and treatment. Kidney Int. 2003;63:1618–31. doi: 10.1046/j.1523-1755.2003.00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B, et al. Kidney disease in patients with HIV infection and AIDS. Infect Dis. 2008;47:1449–57. doi: 10.1086/593099. [DOI] [PubMed] [Google Scholar]
- 5.Herman ES, Klotman PE. HIV-associated nephropathy: epidemiology, pathogenesis, and treatment. Semin Nephrol. 2003;23:200–8. doi: 10.1053/snep.2003.50018. [DOI] [PubMed] [Google Scholar]
- 6.Pardo V, Aldana M, Colton RM, Fischl MA, Jaffe D, Moskowitz L, et al. Glomerular lesions in the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:429–34. doi: 10.7326/0003-4819-101-4-429. [DOI] [PubMed] [Google Scholar]
- 7.Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669–73. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 8.Gardenswartz MH, Lerner CW, Seligson GR, Zabetakis PM, Rotterdam H, Tapper ML, et al. Renal disease in patients with AIDS: a clinicopathologic study. Clin Nephrol. 1984;21:197–204. [PubMed] [Google Scholar]
- 9.Bourgoignie JJ. Renal complications of human immunodeficiency virus type 1. Kidney Int. 1990;37:1571–84. doi: 10.1038/ki.1990.151. [DOI] [PubMed] [Google Scholar]
- 10.Bourgoignie JJ, Ortiz-Interian C, Green DF. The epidemiology of HIV associated nephropathy. In: Hatano M, editor. Nephrology. Tokyo: Springer-Verlag; 1991. pp. 484–92. [Google Scholar]
- 11.Rao TK. Clinical features of HIV associated nephropathy. Kidney Int. 1991;40:S13–8. [PubMed] [Google Scholar]
- 12.Mazbar SA, Schoenfeld PY, Humphreys MH. Renal involvement in patients infected with HIV: Experience at San Francisco General Hospital. Kidney Int. 1990;37:1325–30. doi: 10.1038/ki.1990.118. [DOI] [PubMed] [Google Scholar]
- 13.Noch D, Glotz D, Dosquet P, Pruna A, Guettier C, Weiss L, et al. Renal disease associated with HIV infection: a multicentric study of 60 patients from Paris hospitals. Nephrol Dial Transplant. 1993;8:9–11. doi: 10.1093/oxfordjournals.ndt.a092263. [DOI] [PubMed] [Google Scholar]
- 14.Connolly JO, Weston CE, Hendry BM. HIV-associated renal disease in London hospitals. QJM. 1995;88:627–34. [PubMed] [Google Scholar]
- 15.Naaz I, Wani R, Najar MS, Bandey K, Baba KM, Jeelani H. Collapsing glomerulopathy in an HIV-positive patient in a low-incidence belt. India J Nephrol. 2010;20:211–3. doi: 10.4103/0971-4065.73451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah I. Nephrotic proteinuria and renal involvement in HIV-infected children. Indian J Sex Transm Dis. 2011;32:111–3. doi: 10.4103/2589-0557.85416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz A, Bargman JM, Miller DC, Guo JW, Ghali VS, Schoeneman MJ. IgA nephritis in HIV-positive patients: a new HIV-associated nephropathy? Clin Nephrol. 1992;38:61–8. [PubMed] [Google Scholar]
- 18.Han TM, Naicker S, Ramdial PK. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 2006;69:2243–50. doi: 10.1038/sj.ki.5000339. [DOI] [PubMed] [Google Scholar]
- 19.Winston JA, Klotman ME, Klotman PE. HIV-associated nephropathy is a late, not early, manifestation of HIV-1 infection. Kidney Int. 1999;55:1036–40. doi: 10.1046/j.1523-1755.1999.0550031036.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SK, Mamlin BW, Johnson CS, Dollins MD, Topf JM, Dubé MP, et al. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61:1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 21.Crowley ST, Cantwell B, Abu-Alfa A, Rigsby MO. Prevalence of persistent proteinuria in HIV-infected outpatients and lack of correlation with viral load. Clin Nephrol. 2001;55:1–6. [PubMed] [Google Scholar]
- 22.Varma PP, Prasher PK, Deshpande GU, Mani NS, Nema SK, Sayal SK. Spectrum of renal lesions in HIV patients. J Assoc Physicians India. 2000;48:1151–4. [PubMed] [Google Scholar]
- 23.Emem CP, Arogundade F, Sanusi A, Adelusoia K, Wokoma F, Akinsola A. Renal disease in HIV-seropositive patients in Nigeria: an assessment of prevalence, clinical features and risk factors. Nephrol Dial Transplant. 2008;23:741–6. doi: 10.1093/ndt/gfm836. [DOI] [PubMed] [Google Scholar]
- 24.Praditpornsilpa K, Napathorn S, Yenrudi S, Wankrairot P, Tungsaga K, Sitprija V. Renal pathology and HIV infection in Thailand. Am J Kidney Dis. 1999;33:282–6. doi: 10.1016/s0272-6386(99)70301-x. [DOI] [PubMed] [Google Scholar]
- 25.Haas M, Kaul S, Eustace JA. HIV-associated immune complex glomerulonephritis with “lupus-like” features: A clinicopatologic study of 14 cases. Kidney Int. 2005;67:1381–90. doi: 10.1111/j.1523-1755.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Nochy D, Glotz D, Dosuet P. Renal lesions associated with HIV: North American vs European experience. In: Gruenfeld JF, Bach H, editors. Advances in nephrology. St. Louis: Mosby; 1992. p. 269. [PubMed] [Google Scholar]
- 27.Casanova S, Mazzucco G, Barbiano di Belgiojoso G, Motta M, Boldorini R, Genderini A, et al. Pattern of glomerular involvement in human immunodeficiency virus-infected patients: an Italian study. Am J Kidney Dis. 1995;26:446–53. doi: 10.1016/0272-6386(95)90490-5. [DOI] [PubMed] [Google Scholar]


