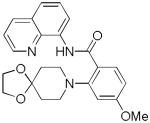
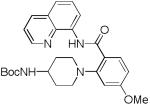
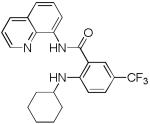
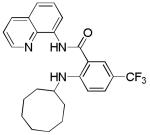
Table 3.
Copper-Catalyzed Amination of 8-Amino-quinoline Amides[a]

| entry | amine | product | yield, % |
|---|---|---|---|
| 1 | HNMeBn |
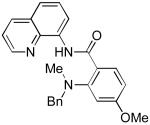
|
82 |
| 2 | HNMePr |
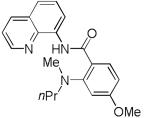
|
74 |
| 3 | 4-EtO2C- piperidine |
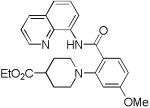
|
69 |
| 4 | 4-NC- piperidine |
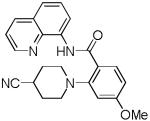
|
71 |
| 5 | Ethylene ketal of 4-keto- piperidine |
|
82 |
| 6 | 4-BocNH- piperidine |
|
83 |
| 7 | Cyclohexyl- amine |
|
40 |
| 8 | Cyclooctyl- amine |
|
52 |
| 9 | Neopentyl- amine |
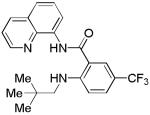
|
32 |
| 10[b] | n-C12H25NH2 |
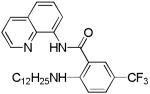
|
20 |
Scale: 0.5 mmol, 2 mL NMP, 2 equiv NMO, 2 equiv amine, 12-25 mol% Cu(OAc)2, 12-25 mol% Ag2CO3, 110 °C, 12 h. Yields are isolated yields.
Pyridine solvent. Please see Supporting Information for details.
