Abstract
Background:
Cigarette smoke and smoking-induced inflammation decrease cystic fibrosis transmembrane conductance regulator (CFTR) activity and mucociliary transport in the nasal airway and cultured bronchial epithelial cells. This raises the possibility that lower airway CFTR dysfunction may contribute to the pathophysiology of COPD. We compared lower airway CFTR activity in current and former smokers with COPD, current smokers without COPD, and lifelong nonsmokers to examine the relationships between clinical characteristics and CFTR expression and function.
Methods:
Demographic, spirometry, and symptom questionnaire data were collected. CFTR activity was determined by nasal potential difference (NPD) and lower airway potential difference (LAPD) assays. The primary measure of CFTR function was the total change in chloride transport (Δchloride-free isoproterenol). CFTR protein expression in endobronchial biopsy specimens was measured by Western blot.
Results:
Compared with healthy nonsmokers (n = 11), current smokers (n = 17) showed a significant reduction in LAPD CFTR activity (Δchloride-free isoproterenol, −8.70 mV vs −15.9 mV; P = .003). Similar reductions were observed in smokers with and without COPD. Former smokers with COPD (n = 7) showed a nonsignificant reduction in chloride conductance (−12.7 mV). A similar pattern was observed for CFTR protein expression. Univariate analysis demonstrated correlations between LAPD CFTR activity and current smoking, the presence of chronic bronchitis, and dyspnea scores.
Conclusions:
Smokers with and without COPD have reduced lower airway CFTR activity compared with healthy nonsmokers, and this finding correlates with disease phenotype. Acquired CFTR dysfunction may contribute to COPD pathogenesis.
The public health impact of COPD continues to increase, now accounting for > $40 billion in annual health-care costs1 and surpassing stroke as the third leading cause of death in the United States.2 At present, there are no effective therapies for the mucus stasis characteristic of the disease, although its presence has been associated with accelerated loss of lung function and mortality.3,4 This is due to multiple complex mechanisms and the absence of a potent and bioavailable drug to target these pathways.5
Small airway mucus obstruction in COPD leads to a pathologic cascade involving bacterial colonization, chronic neutrophilic inflammation, and recurrent exacerbations that are reminiscent of cystic fibrosis (CF) lung disease.6‐8 The phenotypic similarity between COPD and CF is most pronounced in those with symptoms of chronic bronchitis in whom a relatively high incidence of bronchiectasis has been reported9; however, the overproduction, poor clearance, and accumulation of hyperviscous mucus within the small airways is also present in patients with an emphysema-dominant phenotype.3,4
An improved understanding of the role of CF transmembrane conductance regulator (CFTR) in the maintenance of normal epithelial function has revealed that mild and variable CFTR mutations play a causative role in a number of diseases not classically associated with CF.10 With the clinical validation of potent modulators of CFTR ion channel activity,11 there is considerable interest in examining the role of CFTR dysfunction in the pathogenesis of other diseases not previously believed to result from reduced epithelial ion transport, including COPD.
Our laboratory and others have reported that cigarette smoke exposure reduces CFTR expression, function, and mucociliary transport12‐15 in vitro and that smokers with and without COPD exhibit reduced CFTR-dependent nasal potential difference (NPD) in the upper airway.15 However, CFTR activity has not been previously measured in the lungs of individuals with COPD. We hypothesized that CFTR dysfunction would be present in the lower airway of individuals with COPD and that the abnormality would correlate with the presence of chronic bronchitis and markers of disease severity.
Materials and Methods
Study Subjects and Study Protocol
The study was approved by the University of Alabama at Birmingham Institutional Review Board (approval number F090626003), and all patients provided written informed consent. Patients aged 40 to 80 years were categorized into four subgroups by smoking status and the presence or absence of airflow obstruction (FEV1/FVC below the lower limit of normal for age, race, sex, and height). A minimum of 10 pack-years tobacco use was required for patients with COPD, and abstinence was confirmed with serum cotinine levels. Former smokers were abstinent for ≥ 1 year. Exclusion criteria were asthma, bronchiectasis, or other lung disease and any change in respiratory medications or respiratory illness in the month prior to enrollment.
Study Assessments
Spirometry and Exercise Testing:
Postbronchodilator spirometry and 6-min walk tests were performed according to American Thoracic Society standards.16,17
Questionnaires:
Dyspnea was assessed with the Modified Medical Research Council scale.18 Subjects were queried about the presence of chronic bronchitis on the basis of the American Thoracic Society/Medical Research Council definition of cough and sputum present most days of 3 months in 2 consecutive years. The severity of bronchitic symptoms was assessed with the Breathlessness, Cough, and Sputum Scale (BCSS).19
NPD Measurements:
NPD studies were performed according to the description by Solomon et al20 with Electronic Data Capture (ADInstruments), KCl calomel electrodes (Thermo Fisher Scientific Inc), and 3% agar nasal catheter and reference bridges.15,21
Lower Airway Potential Difference Measurements:
During bronchoscopy under conscious sedation with limited topical analgesia (1% lidocaine to the vocal cords and right mainstem bronchus), lower airway potential difference (LAPD) measurements were performed to quantify CFTR activity in the lower airway (Fig 1). The equipment and conditions for measuring bioelectric changes across the airway mucosa were based on the standard NPD protocol adapted to the bronchoscopic technique, including use of agar-filled PE90 tubing for the probing electrode and limiting perfusion solutions to Ringer, Ringer plus amiloride (100 μM), and chloride-free gluconate with amiloride plus isoproterenol (100 μM).22 A stable potential with the mean value of a 10-s scoring interval after perfusion of each solution was recorded by a blinded investigator. All measures were made at the lingula outlet after airway inspection.
Figure 1.
Bronchoscopic placement of the lower airway potential difference catheter. A, Supraglottic view. B, View from position in the lingual outlet.
BAL:
BAL (125 mL saline) was performed immediately after LAPD measurements in the lingula.
CFTR Protein Expression in Endobronchial Biopsy Specimens:
After LAPD measurements and BAL, six endobronchial biopsy specimens were obtained from second- through fifth-order bifurcations in the lower lobes and were flash frozen in liquid nitrogen. CFTR protein expression was estimated by Western blot with a 1:1 mixture of 570 and 596 monoclonal anti-CFTR antibodies.23‐25
Human Neutrophil Elastase Assay:
To estimate the activity of human neutrophil elastase (HNE) in BAL supernatants, InnoZyme Human Neutrophil Elastase Immunocapture Activity assay kits (Calbiochem-EMD Millipore) were used.26
Inflammatory Cytokine Assay:
BAL supernatants were analyzed for the presence of inflammatory mediators by MILLIPLEX map Human Cytokine/Chemokine Premixed 42 Plex assay kits (EMD Millipore).
Genetic Analysis:
CFTR genetic testing was performed at a commercially accredited facility (Medical Genetics Laboratories at Baylor College of Medicine, Houston, Texas) and included the 23 mutations recommended by the American College of Medical Genetics. Patients with any CF-causing allele were excluded.
Statistical Analysis
All measures of CFTR activity and expression were analyzed with descriptive statistics. Results between groups were compared by analysis of variance, and P < .05 was considered significant. Sample size calculations were based on prior experience with NPD and indicated that enrollment of eight patients per group would provide 80% power to detect a 5-mV difference in LAPD (Δchloride-free plus isoproterenol) compared with healthy nonsmokers. Pearson correlations were performed to examine the relationship between clinical characteristics and LAPD-measured CFTR activity as well as between NPD and LAPD. Univariate linear regression was used to determine whether clinical parameters or inflammatory markers were associated with LAPD.
Results
Study Subjects
We enrolled 48 patients of whom eight did not meet spirometric inclusion criteria or were excluded because of comorbid illnesses that increased the risk of bronchoscopy complications (e-Fig 1 (471.1KB, pdf) ). Of the remaining 40 patients, 35 had acceptable LAPD tracings. Tracings were not available for five patients because of technical issues with the perfusion catheter (n = 3), leakage of perfusion fluids (n = 1), or patient intolerance of bronchoscopy (n = 1). Three patients (two COPD smokers and one healthy nonsmoker) underwent repeat testing to assess the within-subject reproducibility of LAPD; the mean values of the two independent measures were used in these cases. There were no instances of clinically significant bronchospasm, hypoxemia, or bleeding. Subject groups were well matched for age, although there were numerically more women among healthy nonsmokers and fewer African Americans among COPD smokers (Table 1). A range of lung function was observed in patients with COPD (FEV1, 35%-82% predicted), and active smokers were more likely to have chronic bronchitis (P < .001).
Table 1.
—Patient Characteristics
| Characteristic | Healthy Nonsmokers (n = 11) | Healthy Smokers (n = 10) | Smokers With COPD (n = 7) | Former Smokers With COPD (n = 7) |
| Age, y | 58 (41-74) | 50 (45-62) | 54 (52-63) | 66 (59-70) |
| Female sex | 8 (73) | 3 (30) | 1 (14) | 2 (29) |
| African American race | 6 (55) | 7 (70) | 1 (14) | 5 (71) |
| BMI, kg/m2 | 31 (22-43) | 30 (24-38) | 25 (21-41) | 29 (21-54) |
| FEV1 % predicted | 97 (90-109) | 90 (85-128) | 48 (36-82) | 65 (43-73) |
| FEV1/FVC | 0.79 (0.72-0.86) | 0.78 (0.71-0.88) | 0.43 (0.36-0.68) | 0.53 (0.41-0.63) |
| Pack-y | 0 (0-0) | 33 (12-54) | 52.5 (35-147) | 45 (39-80) |
| Chronic bronchitis | 0 (0) | 5 (50) | 5 (71) | 1 (14) |
| BCSS | 0 (0-0) | 0.5 (0-4) | 5 (2-9) | 1 (0-7) |
| MMRC dyspnea score | 0 (0-1) | 1 (0-2) | 1 (0-3) | 2 (0-3) |
| Exacerbation in prior year | 0 (0) | 1 (10) | 1 (14) | 2 (29) |
| 6-min walk, m | 417 (349-457) | 372 (146-503) | 303 (253-480) | 330 (137-388) |
| BODE index | N/A | N/A | 3 (1-4) | 2 (1-5) |
Data are presented as median (range) or No. (%). BCSS = Breathlessness, Cough, and Sputum Scale; BODE = BMI, obstruction, dyspnea, exercise tolerance; MMRC = Modified Medical Research Council; N/A = not applicable.
Measures of CFTR Activity in the Lower Airway
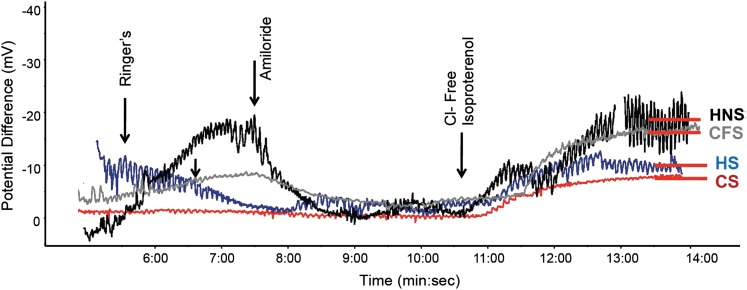
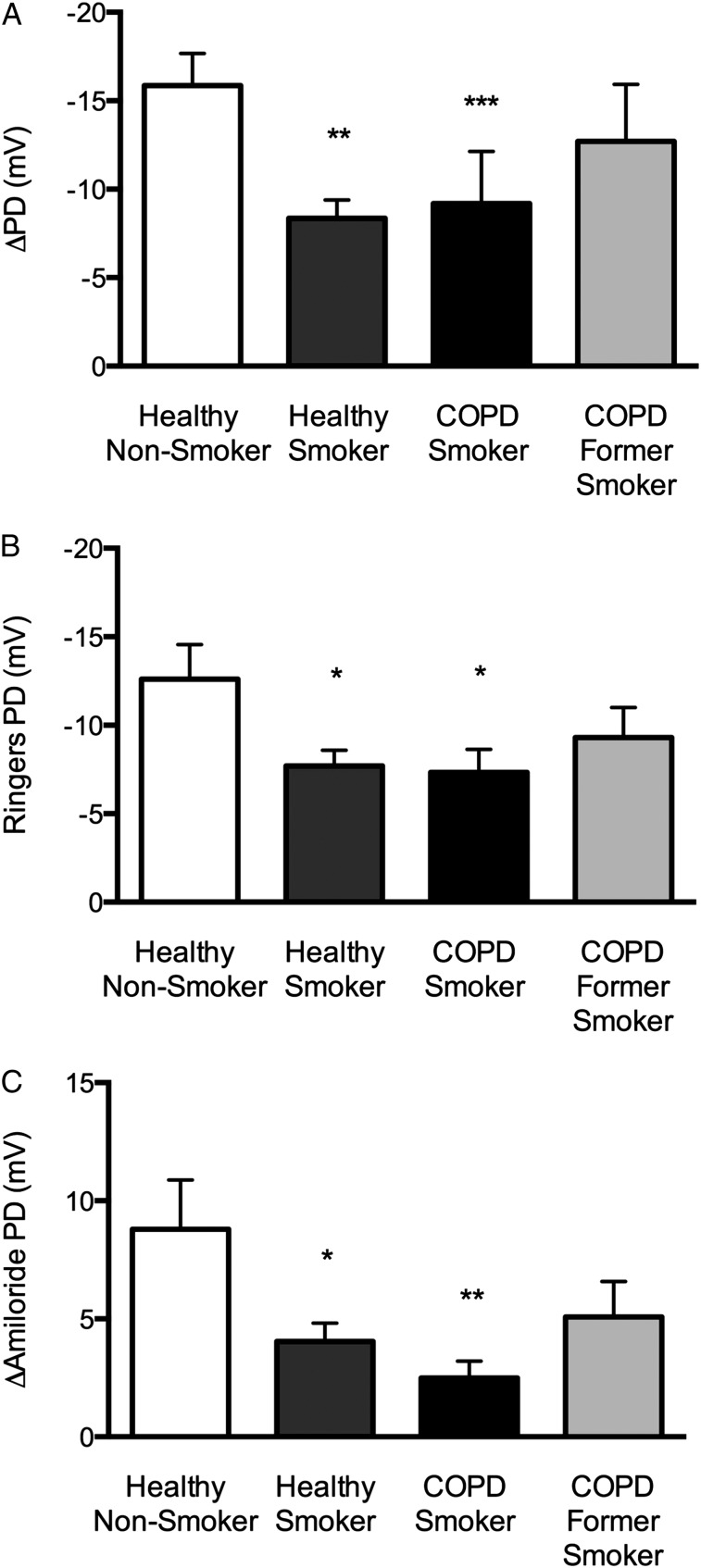
Representative LAPD tracings are shown in Figure 2 and demonstrated the expected change in potential difference in normal control patients with sequential perfusion of Ringer solution, Ringer solution plus amiloride, and chloride-free isoproterenol. Healthy smokers and COPD smokers showed a reduced response to chloride-free isoproterenol, whereas COPD former smokers showed an intermediate response. A significant difference in CFTR-dependent chloride transport (Δchloride-free isoproterenol) was observed among the four patient groups (Fig 3A). Healthy smokers showed a significant reduction in Δchloride-free isoproterenol compared with normal control patients (−8.35 ± 3.10 mV vs −15.90 ± 5.73 mV; P < .002), and a similar decrement was observed in smokers with COPD (−9.19 ± 7.21 mV; P = .054). Former smokers with COPD showed a modest reduction in chloride transport, although this finding was not statistically significant (−12.70 ± 7.88 mV). All patients with COPD showed a trend toward reduced chloride transport compared with healthy nonsmokers (−10.90 ± 7.75 mV; P = .09), an effect mediated by greater variability among COPD former smokers. Two smokers with COPD and one healthy nonsmoker underwent two bronchoscopic LAPD measurements during procedures occurring > 1 month apart. The mean difference in Δchloride-free isoproterenol between the two measurements was 1.43 ± 3.59 mV, which compared favorably to prior NPD studies.20,23 LAPD also revealed abnormalities in estimates of sodium transport, including reduced baseline (Fig 3B) and amiloride-sensitive (Fig 3C) potential differences in healthy smokers and COPD smokers compared with healthy nonsmokers.
Figure 2.
Lower airway potential difference in smokers with and without COPD. Representative lower airway potential difference tracings for each disease group. The start of each perfusion solution is marked with an arrow. Stable potential difference achieved after chloride-free isoproterenol perfusion is denoted with a red bar. High-frequency (0.2-0.5 Hz) noise most visible at the end of the tracing represents artifact caused by respiration. CFS = COPD former smoker; CS = COPD smoker; HNS = healthy nonsmoker; HS = healthy smoker.
Figure 3.
Ion transport activity in smokers with and without COPD. A, Cystic fibrosis transmembrane conductance regulator (CFTR) activity estimated in the lower airway by the change in PD following perfusion with chloride-free isoproterenol. **P < .005; ***P = .054. B, Baseline potential difference as determined by the stable PD following perfusion with Ringer solution. Baseline PD was lower in both healthy smokers (−7.71 ± 0.88) and COPD smokers (−7.33 ± 1.30) than in healthy nonsmokers (−12.61 ± 1.94). *P < .05. C, Amiloride-sensitive PD determined by the change in PD with amiloride perfusion. Amiloride-sensitive PD was lower in healthy smokers (4.04 ± 0.78 mV) and COPD smokers (2.49 ± 0.72 mV) than in healthy nonsmokers (8.79 ± 2.09 mV); COPD former smokers again were intermediate but not statistically different from healthy control patients (5.08 ± 1.50). *P < .05; **P < .005. PD = potential difference.
CFTR Protein Expression
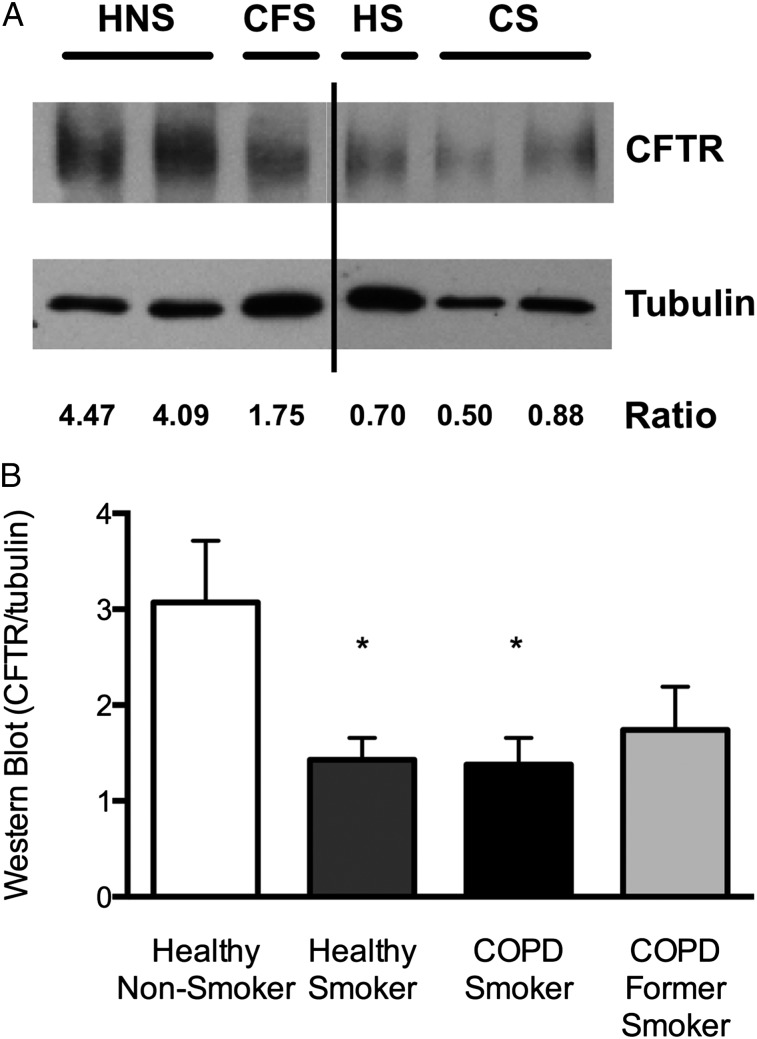
The reduction in CFTR activity in smokers with and without COPD was supported by suppressed CFTR protein expression in endobronchial biopsy specimens (Fig 4). Concordant with functional studies, COPD former smokers had intermediate expression.
Figure 4.
CFTR expression in the lower airway as determined by Western blot analysis. A, Representative blot demonstrating CFTR C-band expression compared with tubulin loading control. The densitometry ratio for each respective lane is shown. B, Summary data of that shown in A. *P < .05. See Figure 2 and 3 legends for expansion of abbreviations.
Relationship Between CFTR Activity in the Lower Airway and Clinical Status
Univariate analysis of likely contributors to reduced CFTR activity in the lower airway revealed statistically significant associations between chloride transport measured by LAPD and current smoking, the presence of chronic bronchitis, and dyspnea (Table 2). Trends between CFTR activity and BCSS score, lung function, and smoking intensity (pack-years) were also observed. Both BCSS and chronic bronchitis were correlated with current smoking (r = 0.57 [P < .001] and r = 0.39 [P = .02], respectively).
Table 2.
—Univariate Analysis Between Clinical Characteristics and Percent Normal LAPD
| Parameter | β (95% CI) | P Value |
| Age | 1.08 (−0.73 to 2.88) | .23 |
| Female sex | 14.8 (−15.6 to 45.3) | .33 |
| African American race | −3.91 (−34.3 to 26.5) | .80 |
| Current smoking | −37.4 (−64.7 to −10.2) | .01 |
| Smoking intensity | −0.43 (−0.91 to 0.04) | .07 |
| BMI | 0.96 (−1.15 to 3.06) | .36 |
| FEV1% predicted | 48.6 (−12.2 to 109) | .11 |
| FEV1/FVC | 22.1 (−15.0 to 59.1) | .23 |
| Chronic bronchitis | −35.2 (−65.3 to −5.04) | .02 |
| BCSS | −4.73 (−10.4 to 0.89) | .10 |
| MMRC dyspnea score | −18.4 (−33.0 to −3.88) | .01 |
| Exacerbation in prior year | 9.85 (−37.6 to 57.3) | .68 |
| 6-min walk | 0.05 (−0.12 to 0.23) | .53 |
See Table 1 legend for expansion of abbreviations.
Correlation Between CFTR Function in the Nose and Lower Airway
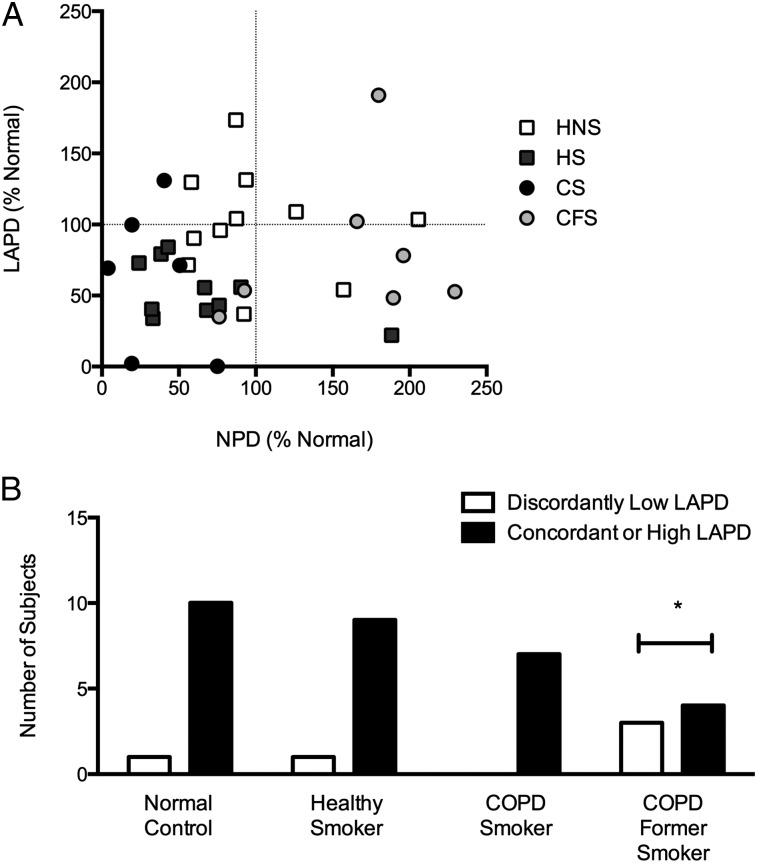
No significant correlation was observed between CFTR-dependent chloride transport measured by NPD and that measured by LAPD (r = 0.17, P = .34) (Fig 5A). LAPD did offer additional information regarding CFTR function, particularly in the COPD former smoker group. As shown in Figure 5B, three of seven former smokers with COPD had an LAPD chloride conductance of less than normal, despite having an NPD that was greater than normal. This pattern was observed in only one of 10 healthy smokers, one of 11 healthy nonsmokers, none of seven COPD smokers, and two of 28 in the other disease groups combined (P < .05).
Figure 5.
Correlation of CFTR function as estimated by NPD and LAPD. Each parameter was calculated as the Δchloride-free isoproterenol perfusion compared with the respective value in the normal control population. A, Correlation between NPD and LAPD. No significant correlation was observed between CFTR-dependent chloride transport measured by NPD compared with LAPD in the overall population (r = 0.17, P = .34) or in healthy nonsmokers (r = −0.13, P = .70). B, Number of patients with a discordantly low LAPD compared with NPD vs the number of patients with a concordant or high LAPD. Discordantly low LAPD was defined as an LAPD below the mean (eg, < 100% of normal) when the NPD was equal to or above the mean (eg, ≥ 100% of normal). *P < .05 by Fisher exact test. LAPD = lower airway potential difference; NPD = nasal potential difference. See Figure 2 and 3 legends for expansion of other abbreviations.
Relationship Between LAPD and the Inflammatory Microenvironment of the Lung
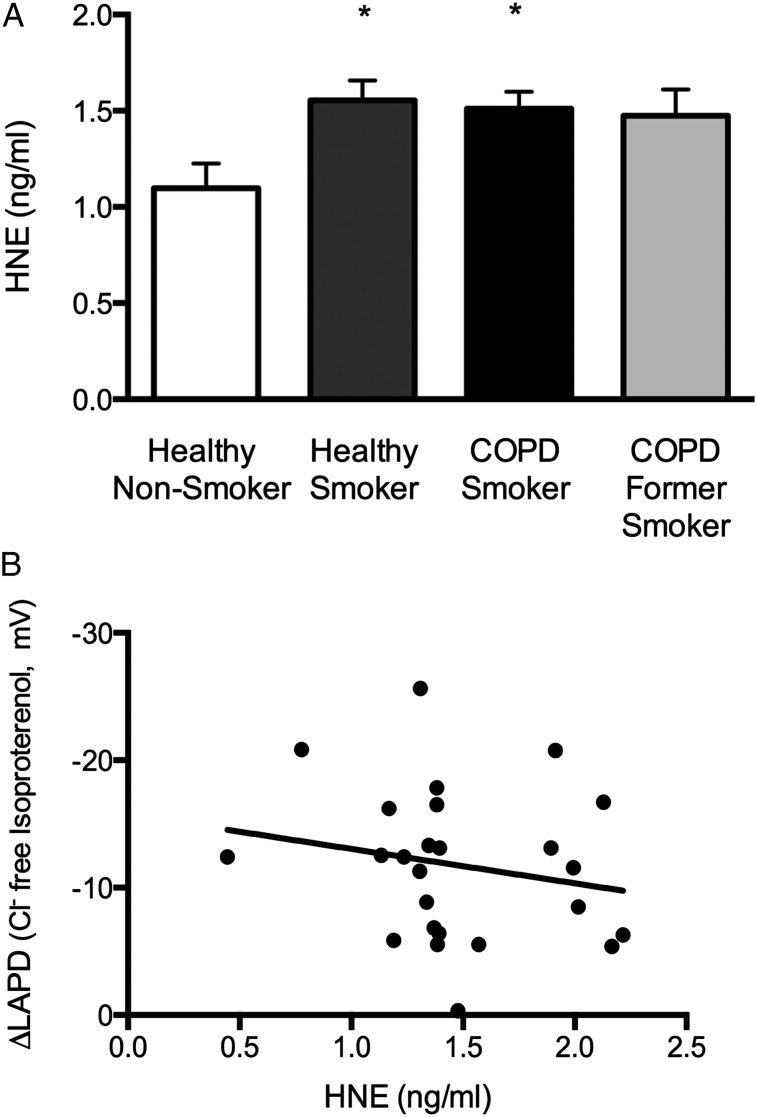
As demonstrated in Figure 6A, HNE activity was significantly increased in smokers with and without COPD compared with healthy nonsmokers (P < .05); activity was also increased in former smokers with COPD, although this difference was not statistically significant. Correlation of HNE levels with CFTR-dependent ion transport as determined by LAPD did not demonstrate a significant relationship between the two (Fig 6B). As summarized in e-Table 1 (471.1KB, pdf) , differences in other BAL inflammatory mediators were observed between groups, although none exhibited a consistent finding that could relate lung inflammation to CFTR activity.
Figure 6.
HNE levels in BAL samples. A, Mean HNE concentration in BAL samples (three 50-μL aliquots/sample) measured by fluorogenic substrate cleavage assay compared with purified substrate. *P < .05. B, Correlation between CFTR activity measured by LAPD (Δchloride-free plus isoproterenol perfusion) and BAL HNE concentration. The regression line was not statistically significant. HNE = human neutrophil elastase. See Figure 3 and 5 legends for expansion of other abbreviations.
Discussion
The results demonstrate that smokers with and without COPD have reduced chloride conductance in the lower airway and that this ion transport abnormality is associated with the presence of chronic bronchitis and dyspnea. These results extend previous findings, suggesting that cigarette smoke has deleterious effects on CFTR activity and epithelial function,12‐15,27 and establish a link between CFTR dysfunction in the lung and clinically relevant COPD symptoms. Detection of this ion transport abnormality was facilitated by the development of a reliable technique for measuring transepithelial LAPD during bronchoscopy under conscious sedation, a method suitable for future research applications, including use in clinical trials to monitor the effect of CFTR modulators.
This study is the first to demonstrate, to our knowledge, that abnormal chloride transport is present in the lungs of smokers with and without COPD and that this defect is associated with clinical phenotype, specifically the presence of chronic bronchitis and the severity of dyspnea, and is consistent with prior NPD data in this population.15 We were unable to demonstrate an independent association between CFTR activity in the lower airway and the severity of bronchitic symptoms because the sample size was relatively small and because there was a strong correlation between each of these parameters and active smoking. Regardless, the results suggest that the pathophysiologic similarities between CF and COPD5 may be partially related to the presence of smoking-induced CFTR dysfunction and that this abnormality may contribute to phenotypic expression in susceptible individuals.
Patients with COPD historically have been grouped into two categories: those exhibiting emphysema (characterized by alveolar destruction and abnormally increased lung compliance) and those with chronic bronchitis (defined by chronic mucus production and characterized by goblet cell hyperplasia and mucus hypersecretion).28 More data have established additional clinical and radiologic features that may aid in the identification of specific COPD subphenotypes with discrete molecular characteristics and, thus, lead to the development of targeted therapies.29,30 The present results suggest that acquired CFTR dysfunction may define a specific subphenotype and contribute to COPD pathogenesis in affected individuals. This concept is supported by the study by Koblizek et al,31 who demonstrated that reduced nasal mucociliary transport is associated with cigarette smoking and correlates with chronic bronchitis symptoms, and suggesting that CFTR dysfunction may underlie these effects.
We have previously reported that subjects with COPD who no longer smoke have normal chloride transport as measured by NPD, suggesting that CFTR function in the upper airway can recover after smoking cessation perhaps because of rapid epithelial cell turnover or other mechanisms.15 The kinetics of the recovery of CFTR activity after smoking cessation have not yet been described but may be different in the lung compared with the nasal airway because of differences in their microenvironments. Given that small airway mucoobstruction and chronic neutrophilic inflammation both persist in the lower airway of patients with COPD, even after smoking cessation,32 the present data indicate that it may be important to directly measure LAPD rather than to rely on NPD measurements as a proxy for CFTR function. As we show, the correlation between NPD and LAPD measures is poor, particularly among former smokers with COPD in whom a partial chloride transport defect may persist specifically in the lung, suggesting that local factors unique to the lower airway could contribute. This partial defect could be explained by reduced epithelial CFTR expression as a result of its continued degradation by HNE, a mechanism recently established by Le Gars et al33 in mice and supported by the overall pattern of the present Western blot analysis and BAL data. Other local pulmonary factors, including hypoxia34 and the unfolded protein response,35 may also affect CFTR activity in the lower airway. Further studies are warranted to determine the relative importance of these pathways and to identify the patient phenotype most at risk for persistent CFTR abnormality despite smoking cessation.
This study has several important limitations, including the relatively small sample size, that did not allow us to determine whether CFTR dysfunction persists in former smokers with COPD or the clinical characteristics independently associated with its presence. In this regard, larger studies; the recruitment of a more homogeneous population in terms of disease severity and expression; and more detailed clinical phenotyping, such as with sputum volume, rheology, or CT scan, to quantify and characterize bronchitis severity are needed.36 Although the study focused on CFTR-dependent chloride transport, we also report significant changes in traditional measures of sodium transport in smokers with and without COPD. This finding contrasts with the typical hyperpolarized baseline potential characteristic of CF22 but is compatible with prior reports of abnormal sodium conductance in the nasal airways of smokers12,15 and may indicate the importance of anion transport as opposed to excess sodium resorption in mediating mucus stasis in COPD. We cannot exclude the possibility that the observed reductions in chloride transport may be partially due to the presence of smoking-induced squamous metaplasia and a consequent reduction in surface CFTR. However, the defect in chloride transport that we report is similar to that found in smoke exposure experiments that used single CFTR channels and human bronchial epithelial cells, and prior studies of CFTR-independent anion transport by NPD in smokers with and without COPD established that disrupted baseline potential difference is not likely a result of nonspecific epithelial injury from smoke exposure.15 Although lidocaine has been reported to affect epithelial sodium transport,37 it is unlikely that this had an impact on the present findings because the dose was minimal, not delivered to the site of LAPD measurement, and was used in all patients, including healthy control patients who showed normal ion transport.
In conclusion, the data establish that acquired CFTR dysfunction is present in the lower airways of smokers with and without COPD and that its presence is associated with clinical symptoms in a subpopulation of affected individuals. Additional studies are needed to determine whether CFTR dysfunction persists in former smokers with COPD and to investigate the relationship between CFTR function in the lower airway and measures of downstream epithelial physiology, including mucociliary transport and whole-lung mucociliary clearance, as well as proof-of-concept trials to examine whether CFTR modulators play a therapeutic role in COPD.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Drs Dransfield and Rowe had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Dransfield: contributed to the study design, study procedures, data analysis, manuscript writing, and manuscript editing.
Dr Wilhelm: contributed to the study procedures, data analysis, and manuscript editing.
Dr Flanagan: contributed to the study procedures, data analysis, and manuscript editing.
Dr Courville: contributed to the study procedures, data analysis, and manuscript editing.
Ms Tidwell: contributed to the study procedures, data analysis, and manuscript editing.
Dr Raju: contributed to the study design, study procedures, data analysis, manuscript writing, and manuscript editing.
Dr Gaggar: contributed to the study design and manuscript editing.
Dr Steele: contributed to the data analysis and manuscript editing.
Dr Tang: contributed to the study procedures, data analysis, and manuscript editing.
Dr Liu: contributed to the data analysis and manuscript editing.
Dr Rowe: contributed to the study design, study procedures, data analysis, manuscript writing, and manuscript editing.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Dransfield has served as a consultant to Boehringer Ingelheim GmbH; GlaxoSmithKline plc; and Ikaria, Inc, and his institution has received research support from the National Institutes of Health; Aeris Therapeutics; AstraZeneca; Boehringer Ingelheim GmbH; Boston Scientific; Centocor Ortho Biotech Inc; Forest Laboratories, Inc; GlaxoSmithKline plc; Ikaria, Inc; MedImmune LLC; Otsuka Pharmaceutical Co, LLC; and Pfizer, Inc. Dr Rowe has served as a consultant for Novartis Corporation and Vertex Pharmaceuticals Incorporated, and his institution has received research support from the National Institutes of Health, Cystic Fibrosis Foundation, Forest Laboratories, Inc; and Vertex Pharmaceuticals Incorporated. Drs Wilhelm, Flanagan, Courville, Raju, Gaggar, Steele, Tang, and Liu and Ms Tidwell have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figure and e-Table can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- BCSS
Breathlessness, Cough, and Sputum Scale
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- HNE
human neutrophil elastase
- LAPD
lower airway potential difference
- NPD
nasal potential difference
Footnotes
Funding/Support: This research is sponsored by the National Institutes of Health [R01 HL105487 to Dr Rowe, R01 HL07783, P30 DK072482, and 5UL1 RR025777] and the Cystic Fibrosis Foundation (CLANCY09Y0 to Dr Rowe and R464-CF). Dr Rowe is supported by American Lung Association Senior Research Fellowship (RT-219427-N).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.National Heart, Lung, and Blood Institute, National Institutes of Health Morbidity and Mortality: 2007 Chart Book on Cardiovascular, Lung and Blood Diseases. Bethesda, MD: National Institutes of Health; 2007:17 [Google Scholar]
- 2.Centers for Disease Control and Prevention National Center for Health Statistics. Deaths: final data for 2007. Final Vital Stat Rep. 2010;58(19). [PubMed] [Google Scholar]
- 3.Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176(5):454-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653 [DOI] [PubMed] [Google Scholar]
- 5.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992-2001 [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(9):991-998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015-1022 [DOI] [PubMed] [Google Scholar]
- 9.Martínez-García MA, Soler-Cataluña JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140(5):1130-1137 [DOI] [PubMed] [Google Scholar]
- 10.Knowles MR, Durie PR. What is cystic fibrosis? N Engl J Med. 2002;347(6):439-442 [DOI] [PubMed] [Google Scholar]
- 11.Ramsey BW, Davies J, McElvaney NG, et al. ; VX08-770-102 Study Group A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173(10):1139-1144 [DOI] [PubMed] [Google Scholar]
- 13.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L894-L902 [DOI] [PubMed] [Google Scholar]
- 14.Savitski AN, Mesaros C, Blair IA, Cohen NA, Kreindler JL. Secondhand smoke inhibits both Cl- and K+ conductances in normal human bronchial epithelial cells. Respir Res. 2009;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloane PA, Shastry S, Wilhelm A, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE. 2012;7(6):e39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187 [DOI] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117 [DOI] [PubMed] [Google Scholar]
- 18.Brooks SM. The evaluation of occupational airways disease in the laboratory and workplace. J Allergy Clin Immunol. 1982;70(1):56-66 [DOI] [PubMed] [Google Scholar]
- 19.Leidy NK, Schmier JK, Jones MK, et al. Evaluating symptoms in chronic obstructive pulmonary disease: validation of the Breathlessness, Cough and Sputum Scale. Respiratory Med. 2003;97(suppl A):S59-S70 [PubMed] [Google Scholar]
- 20.Solomon GM, Konstan MW, Wilschanski M, et al. An international randomized multicenter comparison of nasal potential difference techniques. Chest. 2010;138(4):919-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe SM, Clancy JP, Wilschanski M. Nasal potential difference measurements to assess CFTR ion channel activity. Methods Mol Biol. 2011;741:69-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies JC, Davies M, McShane D, et al. Potential difference measurements in the lower airway of children with and without cystic fibrosis. Am J Respir Crit Care Med. 2005;171(9):1015-1019 [DOI] [PubMed] [Google Scholar]
- 23.Rowe SM, Varga K, Rab A, et al. Restoration of W1282X CFTR activity by enhanced expression. Am J Respir Cell Mol Biol. 2007;37(3):347-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varga K, Goldstein RF, Jurkuvenaite A, et al. Enhanced cell surface-stability of rescued DeltaF508 cystic fibrosis transmembrane conductance regulator by pharmacological chaperones. Biochem J. 2008;410(3):555-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyle LC, Fulton JC, Sloane PA, et al. Activation of cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue. Am J Respir Cell Mol Biol. 2010;43(5):607-616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152(6 pt 1):2111-2118 [DOI] [PubMed] [Google Scholar]
- 27.Clunes LA, Davies CM, Coakley RD, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26(2):533-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedlander AL, Lynch D, Dyar LA, Bowler RP. Phenotypes of chronic obstructive pulmonary disease. COPD. 2007;4(4):355-384 [DOI] [PubMed] [Google Scholar]
- 29.Rennard SI, Vestbo J. The many “small COPDs”: COPD should be an orphan disease. Chest. 2008;134(3):623-627 [DOI] [PubMed] [Google Scholar]
- 30.Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene Study: associated radiologic phenotypes. Radiology. 2011;261(1):274-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koblizek V, Tomsova M, Cermakova E, et al. Impairment of nasal mucociliary clearance in former smokers with stable chronic obstructive pulmonary disease relates to the presence of a chronic bronchitis phenotype. Rhinology. 2011;49(4):397-406 [DOI] [PubMed] [Google Scholar]
- 32.Retamales I, Elliott WM, Meshi B, et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164(3):469-473 [DOI] [PubMed] [Google Scholar]
- 33.Le Gars M, Descamps D, Roussel D, et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med. 2013;187(2):170-179 [DOI] [PubMed] [Google Scholar]
- 34.Guimbellot JS, Fortenberry JA, Siegal GP, et al. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol. 2008;39(5):514-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartoszewski R, Rab A, Twitty G, et al. The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem. 2008;283(18):12154-12165 [DOI] [PubMed] [Google Scholar]
- 36.Kim V, Han MK, Vance GB, et al. ; COPDGene Investigators The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knowles MR, Buntin WH, Bromberg PA, Gatzy JT, Boucher RC. Measurements of transepithelial electric potential differences in the trachea and bronchi of human subjects in vivo. Am Rev Respir Dis. 1982;126(1):108-112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement