Abstract
Molecular clock data indicate that the first zygomycetous fungi occurred on Earth during the Precambrian, however, fossil evidence of these organisms has been slow to accumulate. In this paper, the fossil record of the zygomycetous fungi is compiled, with a focus on structurally preserved Carboniferous and Triassic fossils interpreted as zygosporangium-gametangia complexes and resembling those of modern Endogonales. Enigmatic microfossils from the Precambrian to Cenozoic that have variously been interpreted as, or compared to, zygomycetous fungi are also discussed. Among these, the spherical structures collectively termed ‘sporocarps’ are especially interesting because of their complex investments and abundance in certain Carboniferous and Triassic rocks. Circumstantial evidence suggests that at least some ‘sporocarp’ types represent mantled zygosporangia. Zygomycetous fungi probably were an important element in terrestrial paleoecosystems at least by the Carboniferous.
Keywords: Endogonales, fossil record, morphology, reproductive biology, sporocarp, zygosporangium-gametangia complex
INTRODUCTION
The zygomycetous fungi (formerly Zygomycota) comprise ~1 % of the true fungi; approximately 900 living species have been described (Kirk et al. 2001). They are an ecologically heterogeneous, para- or polyphyletic assemblage of predominantly terrestrial organisms (White et al. 2006, Liu et al. 2009, Liu & Voigt 2010). The vegetative mycelium generally lacks septa except where reproductive units are produced and in regions of old hyphae. Zygomycetous fungi reproduce asexually via non-motile endospores formed in sporangia, sporangiola, or merosporangia, or by the formation of chlamydospores, arthrospores, and yeast cells, and sexually (where documented) by the formation of zygospores following gametangial fusion, or azygospores without prior gametangial conjugation (Benjamin 1979, Benny et al. 2001). Most zygomycetous fungi thrive as saprotrophs, others as parasites of plants, animals, and other fungi (White et al. 2006, Richardson 2009); still others enter into mutualistic associations (mycorrhizae) with plants (Fassi et al. 1969, Walker 1985).
Molecular clock estimates indicate that the first zygomycetous fungi occurred on Earth during the Precambrian, approximately 1.2–1.4 Ga ago (Heckman et al. 2001, Blair 2009); more conservative estimates place the divergence at about 800 Ma (Berbee & Taylor 2001). If these estimates are accurate, zygomycetous fungi were certainly important elements in ancient terrestrial ecosystems. Nevertheless, documented evidence of fossil zygomycetes continues to be rare. Not even the famous Early Devonian Rhynie chert (~410 Ma), which is the single-most important source of information on fossil fungi to date relative to paleoecosystem functioning (Taylor et al. 2004), has produced conclusive evidence of zygomycetous fungi. As a result, efforts in reconstructing the evolutionary history and phylogeny of the zygomycetous fungi or of lineages within this group are to date based exclusively on the analysis of extant members (e.g., White et al. 2006, Liu et al. 2009, Petkovits et al. 2011).
The scarcity of fossil evidence of zygomycetous fungi remains perplexing, especially in light of the fact that habitats conducive to the growth of these organisms, together with depositional environments conducive to their preservation, were available at least by the Paleozoic (see Krings et al. 2012a). The scarcity of reports appears to be related to the nature of the fossil record of fungi in general that typically results in the preservation of isolated parts or stages of the life cycle. Another reason may be that most zygomycetous fungi are saprotrophs. While biotrophic fungi often trigger the formation of host responses, and/or possess specific infection/penetration structures (e.g., appressoria), along with special features facilitating nutrient extraction from the host (e.g., arbuscules, haustoria), saprotrophs do not normally possess special structures that allow for positive recognition as fossils. As a result, far more attention has been directed to date at biotrophic than at saprotrophic fossil fungi. Despite the comments above there is increasing evidence of fossil zygomycetous fungi that indicate an untapped wealth of information and more recently, a new emphasis on their paleodiversity and evolutionary history. This paper compiles the fossil evidence of the zygomycetous fungi, with a focus on structurally preserved remains interpreted as zygosporangium-gametangia complexes. Moreover, we also report on several enigmatic fossils from chert, coal balls, amber, shales, and palynological sampling that have variously been referred to the zygomyceteous fungi. Of the latter, the so-called ‘sporocarps’ are discussed in greater detail because of their interesting morphology and abundance in certain rocks, especially those from the Carboniferous.
MODES OF PRESERVATION
The success of recognising and documenting fossil fungi relies heavily on the mode of preservation and technique(s) used to prepare samples. The most common types of fossil preservation (i.e., impressions, compressions, casts and moulds), with the exception of compressions with preserved cuticle (e.g., see Dilcher 1965, Krings 2001, Hübers et al. 2011), do not normally provide sufficient resolution to detect fungi, let alone to determine their systematic affinities. To date coal balls and chert represent the only sources of compelling evidence of fossil zygomycetes. While coal balls are typically concretions of calcium carbonate, chert deposits generally are an extremely dense microcrystalline or cryptocrystalline type of sedimentary rock. In both the organisms are embedded in the mineral matrix (Taylor et al. 2009). Coal balls and chert preserve not only three-dimensional and structural features of the organisms, but often also details of individual cells and subcellular structures (e.g., chromosomes). As a result of the fidelity of preservation, coal balls and chert provide an optimal matrix from which to extract information about fossil fungi. Although various types of body fossils of fungi and/or indirect evidence of their activities have also been preserved by other modes, including other types of silicification and amber, zygomycetous fungi have not yet been documented from these types of preservation, with a few possible exceptions (see below).
The most appropriate technique to study fossil fungi preserved in coal balls and chert is the standard thin section technique (Taylor et al. 2011), which involves a piece of the chert or coal ball being cemented to a glass slide, thinly sliced, and then ground with an abrasive powder until the section is thin enough to be examined in transmitted light (for details, refer to Hass & Rowe 1999).
IDENTIFICATION
There are several inherent problems that have generally limited our understanding of the fossil record of the fungi. Historically it has been assumed that fungi are extremely delicate organisms with a poor preservation potential, and thus probably not well represented in the rock record. Another reason involved a difficulty in identifying and interpreting fungal remains, in part because of a lack of familiarity of the majority of paleontologists with fungal morphology and systematics. Moreover, the fungal fossil record usually consists of incomplete organisms and/or isolated stages of a life cycle. As a result, direct comparisons between the fossils and modern representatives that could be useful in determining the systematic affinities of the fossils are seldom feasible, and thus render interpretation of fungal remains a challenging area of research. Finally, some of the diagnostic features of the groups are especially difficult to resolve in fossil preparations, in addition to the fact that not all modern characters may have been present in the earliest diverging members of the group.
Most structures formed during the zygomycetous life cycle are non-diagnostic at the level of resolution available with transmitted light. However, mature zygosporangia or zygospores with attached gametangia and/or suspensors, as well as perhaps certain asexual reproductive structures such as the columellate sporangia of the Mucoraceae, appear to be components of the life cycle that lend themselves to preservation in a recognizable form, and thus can be used to positively identify a fossil zygomycete.
FOSSILS INTERPRETED AS OR COMPARED TO ZYGOMYCETOUS FUNGI
Precambrian microfossils
Although molecular clock estimates indicate that the zygomycetous fungi originated in the Precambrian, compelling fossil evidence to corroborate this hypothesis have not been produced to date. Nevertheless, there are a few enigmatic Precambrian microfossils that have been compared with zygomycetous fungi. For example, Hermann (1979) and Hermann & Podkovyrov (2006) describe compressions of irregularly aggregated filaments, globules, and what appear to be copulating filaments from the Lakhanda microbiota (Late Riphean; ~1020–1030 Ma) of the Uchur-Maya Region of south-eastern Siberia as Mucorites ripheicus. The fossils are believed to represent different life cycle stages of a mucoralean zygomycete, in which gametangial fusion and (a-)zygospore formation are virtually identical to that observed in the modern Mucor tenuis. Structures interpreted as sporangiophores were also found. Hermann & Podkovyrov (2006) are convinced that the morphology of the fossils can be used to establish their systematic affinities with the zygomycetes. Slightly older than the Lakhanda fossils are dispersed remains from the Middle Riphean Debengdinskaya Formation (~1200–1300 Ma) of the Olenekskiy uplift in Siberia described by Stanevich et al. (2007). Among these latter fossils are several thick-walled spherical structures (named Lophosphaeridium sp. 1) that have been compared to zygosporangia seen in modern members of the Mucorales.
Another Proterozoic fossil that has been interpreted as representing some level of fungal organization is the Meso/Neoproterozoic (~1600–542 Ma) Tappania, an organism previously described as an acritarch (e.g., Yin 1997, Javaux et al. 2001, Butterfield 2005, Nagovitsin 2009). In this fossil (Fig. 1a), filamentous processes with cross walls form a series of anastomoses surrounding a central vesicle. Butterfield (2005) uses this multicellular level of organisation to suggest that Tappania represents a putative fungus that occupies a position somewhere between the Ascomycota and zygomycetous fungi. Moczydłowska et al. (2011), however, dismiss the fungal affinities of Tappania. Establishing the biological affinities of Tappania, including whether it in fact is fungal, will require more definitive evidence.
Fig. 1.
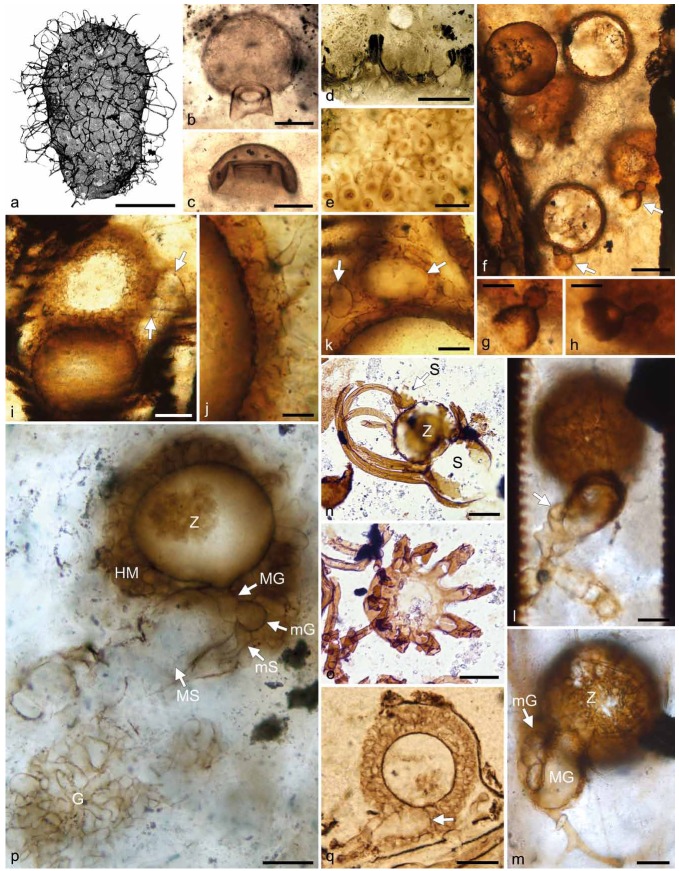
Fossil evidence of zygomycetous fungi (references and further explanations in the text). a. Tappania sp., Lower Neoproterozoic shale, Canada (courtesy N.J. Butterfield). — b, c. Microfossils resembling mucoralean columellae; Lower Devonian Rhynie chert, Scotland. — d, e. Winfrenatia reticulata, Lower Devonian Rhynie chert, Scotland; d. thallus; e. hyphal net enclosing cyanobacterial unicells. — f–h. Fungal reproductive units interpreted as zygosporangia with apposed gametangia, Lower Pennsylvanian coal ball, Great Britain; f. group of specimens, two of which showing paired gametangia (arrows); g, h. gametangial fusion. — i–k. Fungal reproductive units interpreted as mantled zygosporangia with apposed gametangia, Lower Pennsylvanian coal ball, Great Britain; i. two specimens, one showing paired gametangia (arrows); j. investment; k. gametangia or suspensors in cross section (arrows). — l, m. Halifaxia taylorii, Lower Pennsylvanian coal ball, Great Britain; l. ‘smaller element’ and microgametangial branch (arrow); m. mantled zygosporangium (Z), macrogametangium (MG), and microgametangial branch (mG). — n, o. Protoascon missouriensis, Upper Pennsylvanian coal ball, USA; n. large suspensor (S), ornamented zygosporangium (Z), and putative small suspensor (arrow, S); o. suspensor appendages. — p. Jimwhitea circumtecta, Middle Triassic permineralized peat (chert), Antarctica, zygosporangium (Z) enveloped in hyphal mantle (HM), macrogametangium (MG) and macrosuspensor (MS), microgametangium (mG) and microsuspensor (mS), gleba (G). — q. Fungus No. 4, Middle Triassic permineralized peat (chert), Antarctica, mantled zygosporangium subtended by sac-like gametangium (arrow). — Scale bars: a = 100 μm; b, c, i, l–q = 20 μm; d = 1 mm; e, f = 50 μm; g, h, k = 10 μm; j = 5 μm.
The Early Devonian Rhynie chert
The Rhynie chert, an in situ silicified Early Devonian hot spring environment characterised by ephemeral freshwater pools scattered across the landscape, no doubt represents the most famous fossiliferous chert deposit. Within the chert are abundant exquisitely preserved fossil fungi (Taylor et al. 2004), including members of the Chytridiomycota (e.g., Taylor et al. 1992, Krings et al. 2009), Blastocladiomycota (Remy et al. 1994), Glomeromycota (e.g., Taylor et al. 1995, Dotzler et al. 2009), and Ascomycota (Taylor et al. 2005a), as well as representatives of the fungus-like Peronosporomycetes (Taylor et al. 2006, Krings et al. 2012b). However, the Rhynie chert has not produced conclusive evidence of the presence of zygomycetous fungi to date. Those Rhynie chert fungi that historically have been assigned to the zygomycetous fungi (e.g., the AM fungus Glomites rhyniensis; see Taylor et al. 1995) today are accommodated in the Glomeromycota.
It has been suggested that the mycobiont of the lichen-like dual organism Winfrenatia reticulata from the Rhynie chert (Taylor et al. 1997, Karatygin et al. 2009) was a member of the zygomycetous fungi based on the presence of aseptate hyphae and thick-walled, sculptured spores (Taylor et al. 1997). Winfrenatia reticulata consists of a mycelial mat constructed of interwoven hyphae. Along the upper surface of the mat are numerous shallow depressions (Fig. 1d), within which are coccoid unicells that are morphologically similar to certain extant cyanobacteria. Hyphae of the fungus extend into the depressions and become intertwined with the cyanobacteria (Fig. 1e).
Another Rhynie chert fossil that might represent part of a zygomycetous fungus occurs in the form of tiny, globose to subglobose structures, uniform in size and shape and 50–60 μm diam (Fig. 1b). These structures occur singly or in groups dispersed in the chert matrix, close to, but never in, degrading land plant axes and sporangia. Some of the specimens are collapsed, i.e. the proximal half appears deflated with the distal half depressed on top, giving the whole structure an umbrella-like configuration when viewed laterally (Fig. 1c). On the proximal side of each of the spheres is a circular orifice that is surrounded by a conspicuous collar-like structure. The proximal end of the collar appears irregular, suggesting that it may have been mechanically separated. The fossils are morphologically quite similar to columellae seen in members of the extant genus Rhizopus (Mucorales). If this assignment is accurate, then the orifice accordingly represents the attachment site of a sporangiophore, and the collar-like appendage the proximal portion of a peridium that has been repositioned downwards.
Carboniferous and Triassic zygosporangium-gametangia complexes and sporocarps
Carboniferous records
The oldest compelling fossil evidence of zygomycetous fungi occurs in the form of three different types of structurally preserved reproductive units interpreted as (mantled) zygosporangia with apposed gametangia that are preserved in coal balls from the Lower Pennsylvanian (Carboniferous; ~318–311 Ma) of Great Britain.
The first of these fossils occurs in a gymnosperm ovule (Krings & Taylor 2012b). Ten specimens of this reproductive unit have been detected in the space that the nucellus and megaspore would occupy in the seed if preserved. They consist of a smooth-walled near perfect sphere (55 μm diam) to which is attached a hollow, dome-shaped structure that is open at its wide end. Attached to the tip of the dome-shaped structure is a smaller element, which may be more or less spherical, drop- or dome-shaped (arrows in Fig. 1f). This structure, which is 5–8 μm diam, also appears to be open at one end. The lumina of the large sphere and dome-shaped structure, as well as the lumina of the dome-shaped and small element are interconnected (Fig. 1g, h).
The second fossil consists of an assemblage of c. 40 reproductive units that occur in the tracheids of a fragment of degraded wood (Krings & Taylor 2012a). The reproductive units occur singly or in aggregations. Single specimens are spherical to oval in shape and up to 90 μm (95 × 70 μm in oval individuals) diam, while aggregated individuals are more variable in shape. All reproductive units are composed of a central cavity sheathed by a prominent investment (Fig. 1i). The investment (or mantle) is constructed of two different types of elements, with the outer, prominent element composed of hyphae, and the inner element non-hyphal. The outer investment is formed of tightly interlaced hyphae (Fig. 1j); septa are present but appear to be relatively rare. The inner layer is recognizable as a dark line extending along the inner surface of the hyphal mantle. Aggregates of specimens may additionally be surrounded by a confluent meshwork of wide aseptate hyphae. Closely associated with many of the reproductive units are smaller spherical to elongate structures. In most specimens, one associated structure is recognizable (arrows in Fig. 1i), but in some, associated structures occur in pairs (arrows in Fig. 1k). The two associated structures forming a pair appear to be organically connected with each other. Narrow subtending hyphae indicate that the associated structures are not formed as outgrowths of the reproductive units.
The third fossil, which has been formally described as Halifaxia taylorii (Krings et al. In press), occurs in the xylem of a fern axis. The reproductive units (Fig. 1l, m) occur singly, and consist of a sphere subtended by an inflated structure that is termed in the original description informally as a ‘subtending structure’. An irregularly shaped element, termed ‘smaller element’, is found attached to the proximal portion of the subtending structure in some of the specimens. The sphere (Z in Fig. 1m) is 85–90 μm diam and composed of a central cavity surrounded by a hyphal mantle. The subtending structure (MG in Fig. 1m) is sac-like or primarily conical, and in most specimens sheathed by loosely interwoven hyphae. A smaller element (mG in Fig. 1m), which lacks a hyphal investment, clasps the proximal portion of the subtending structure, and then produces one stout branch that extends further up along the outer surface of the subtending structure. The tip of this branch appears to fuse laterally with the subtending structure. A transverse septum separates the distal portion of the branch from the rest (arrow in Fig. 1l).
All three reproductive units have been interpreted as zygosporangium-apposed gametangia complexes. The spherical component is believed to represent the zygosporangium, which, in two of the fossils, is covered by a hyphal mantle. The associated structures accordingly represent the two gametangia, each subtended by a suspensor. In two of the fossils, the gametangia differ from each other in size, and thus are termed macro- and microgametangium. The condition seen in the fossils closely corresponds to that in certain modern representatives of the Endogonaceae (see Bucholtz 1912, Thaxter 1922, Yao et al. 1996). Moreover, it has been observed that in certain Endogonaceae the gametangium walls increase in thickness after gametangial fusion, and thus may remain intact even until zygosporangium maturation (e.g., Bucholtz 1912: 162). This observation may explain why both the large and small associated structure in the fossil found within a gymnosperm ovule are open at one end (Fig. 1f–h): The open ends would correspond to the attachment sites of the gametangia to the subtending suspensors, which do not have secondarily thickened walls, and thus rapidly disintegrate following maturation of the zygosporangium and zygospore. Adding support to this interpretation is the fact that the configuration exhibited by these fossils is virtually identical to that seen in several of the zygosporangia with attached paired gametangia of extant Endogone species (e.g., Yao et al. 1996: pl. 4, f. 30, Błaszkowski et al. 1998: f. 5, 2004: f. 8). A structural feature of Halifaxia taylorii that does not occur in Endogonaceae is the smaller element subtending the microgametangial branch and clasping around the proximal portion of the subtending structure (Fig. 1l). However, a somewhat similar feature has been reported as occurring during sexual reproduction in Mortierella capitata, in which the microprogametangium initially develops a branched structure that entwines densely around the elongating, club-shaped macroprogametangium (Degawa & Tokumaso 1997).
A geologically slightly younger fossil interpreted as a zygosporangium-gametangia complex is Protoascon missouriensis, an assemblage of fungal reproductive units that occur in a seed preserved in a coal ball from the Middle Pennsylvanian (~311–307 Ma) of North America (Taylor et al. 2005b). Each of the reproductive units consists of a pair of conjoined spheroids 50–150 μm diam, in which the distal spheroid (Z in Fig. 1n) is thick-walled and ornamented, while the proximal spheroid is relatively thin-walled (S in Fig. 1n). Up to 12 filamentous appendages arise from near the apex of the proximal spheroid and envelop the distal spheroid (Fig. 1o). Each pair of spheroids measures approximately 250 μm from the base of the proximal spheroid to the tip of the enclosing appendages. It appears that, in one of the specimens, a second, smaller sphere (arrow S in Fig. 1n) is attached to the ornamented sphere in opposite position to the proximal sphere.
As the name suggests, Protoascon missouriensis was initially thought to be a member of the Ascomycota (Batra et al. 1964), but later reinterpreted as belonging to the Chytridiomycota (Baxter 1975). However, subsequent studies (Pirozynski 1976, Taylor et al. 2005b) have reinterpreted the proximal spheroid and associated appendages as a suspensor of a zygomycete and the distal, ornamented spheroid containing a single sphere as an azygo- or zygosporangium like those seen in many modern zygomycetes.
Although the occurrence of fossils of zygomycetous fungi in great numbers in the Carboniferous has been postulated 100 years ago by the British paleontologist R.C. McLean (1912), only four putative Carboniferous representatives of this group of fungi have been documented. It is interesting to note that all Carboniferous zygomycetes described to date occur within the confines of plant parts. This is unusual since most modern zygomycetes produce zygospores aerially, on or in the soil, or on organic debris (Benny et al. 2001). As to whether the occurrence of the Carboniferous zygosporangium-gametangia complexes within plant parts represents a preservation bias in which only those specimens protected by plant tissue are preserved in a recognizable form, or reflects some life history strategy of zygomycetous fungi in the Carboniferous cannot be determined.
Triassic records
Probably the most persuasive fossil representative of the Endogonaceae has been discovered in permineralized peat from the Middle Triassic (~245–228 Ma) of Antarctica and formally described as Jimwhitea circumtecta (Krings et al. 2012a). This fossil (Fig. 1p) consists of a spheroid born on an inflated, sac-like structure to which is attached a smaller globose element subtended by a distally widened hypha. The spheroid (Z in Fig. 1p) is 85 μm diam and composed of a central cavity surrounded by a prominent, two-layered mantle, with the outer layer composed of hyphae (HM in Fig. 1p), and the inner layer non-hyphal. Subtending the spheroid is a smooth-walled sac-like structure; a direct connection exists between the central cavity of the spheroid and the lumen of the sac-like structure. The distal portion of the sac-like structure (MG in Fig. 1p) is separated from the rest (MS in Fig. 1p) by a septum. Physically connected to the tip region of the sac-like structure is a much smaller globose element (mG in Fig. 1p), which is subtended by a hypha-like structure (mS in Fig. 1p). The lumina of the globose element and sac-like structure are interconnected. Where the proximal end of the sac-like structure was at one time (not preserved) occurs a patch of a conspicuous meshwork of multi-branched, irregularly shaped, tightly interlaced hyphae (G in Fig. 1p).
In Jimwhitea circumtecta the spheroidal component is viewed as a zygosporangium, with the hyphal investment representing the mantle and the inner, non-hyphal layer the sporangiothecium. The sac-like structure accordingly represents the macrogametangium, which is subtended by a macrosuspensor (with a septum between the two structures), while the small globose element attached to the tip of the sac-like structure is interpreted as a microgametangium subtended by a microsuspensor. The meshwork of tightly interlaced hyphae at the proximal end of the macrosuspensor likely represents the gleba that gives rise to the gametangia.
Another fossil from the Middle Triassic of Antarctica that is quite similar to Jimwhitea circumtecta has been described and informally named Fungus No. 4 by White & Taylor (1989b). This fossil (Fig. 1q) consists of a mantled sphere (~60 μm diam) subtended by an inflated, sac-like structure (arrow in Fig. 1q), which likely represents the macrogametangium and macrosuspensor. Evidence of gametangial fusion, however, is lacking.
Co-occurring with Jimwhitea circumtecta in the same chert block is a sporocarp portion that is bounded on the outside by a narrow peridium or pseudoperidium (Krings et al. 2012a). The sporocarp contains 12 sporangia/spores, which are embedded in a gleba of irregularly swollen, thin-walled hyphae. The individual sporangia/spores are (sub)globose or ovoid and up to 60 μm diam. Some sporangia/spores are surrounded by what appears to be a developing hyphal mantle that is incomplete (i.e. not traceable around the entire sporangium). Several of the sporangia/spores are physically connected to sac-like structures. The sporangia/spores contained in the sporocarp are approximately the size of the zygosporangium of J. circumtecta. Moreover, several of the sporangia in the sporocarp are borne on sac-like structures, which are interpreted as gametangia/suspensors. Gametangial fusion, however, has not been observed. In addition, the patch of interlaced hyphae interpreted as a gleba closely associated with J. circumtecta (G in Fig. 1p) is structurally similar to the sporocarp gleba. All these correspondences strongly suggest that the sporocarp also belongs to J. circumtecta.
Two additional sporocarps containing sporangia/spores with suggested affinities to the Endogonales have been described from the Middle Triassic of Antarctica and informally named Fungus No. 2 and Fungus No. 3 (White & Taylor 1989b). Fungus No. 2 (Fig. 2a) is 600 × 1000 μm diam, and composed of numerous spores surrounded by a mycelial peridium composed of interwoven hyphae. Individual spores are globose and 60–67 μm diam; some possess a spherical or drop-shaped associated structure 18–20 μm diam (arrows in Fig. 2b, c), which has been interpreted by these authors as a suspensor cell. Fungus No. 3 (Fig. 2d) is similar to Fungus No. 2. However, individual spores are characterised by a prominent mantle composed of tightly interlaced hyphae (Fig. 2e). The sporocarp portion co-occurring with J. circumtecta differs from both sporocarps described by White & Taylor (1989b) with regard to peridium thickness, which is up to 180 μm in Fungus No. 2 and up to 90 μm in Fungus No. 3. Moreover, a gleba has not been reported in either Fungus No. 2 or Fungus No. 3.
Fig. 2.
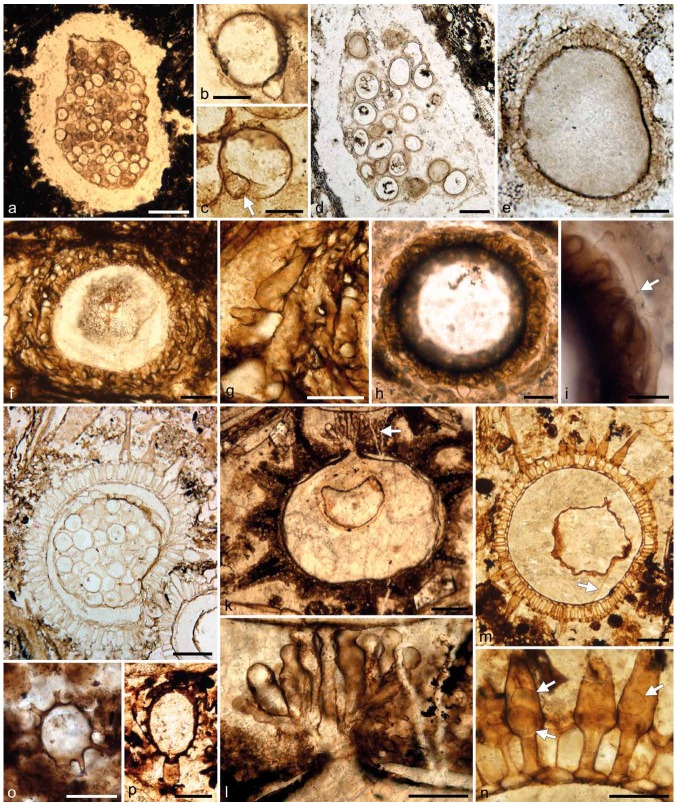
Fossil evidence of zygomycetous fungi (references and further explanations in the text). a–c. Fungus No. 2, Middle Triassic permineralized peat (chert), Antarctica; a. sporocarp; b, c. spores with drop-shaped associated structure (arrow in c). — d, e. Fungus No. 3, Middle Triassic permineralized peat (chert), Antarctica; d. sporocarp; e. mantled zygosporangium. — f, g. Mycocarpon sp., Lower Pennsylvanian coal ball, Great Britain; f. specimen showing prominent hyphal investment; g. detail of investment. — h, i. Mycocarpon cinctum, Middle Mississippian chert, France; h. morphology; i. investment composed of inner layer of radially oriented hyphal segments and peripheral septate (arrow) hyphae extending along circumference of structure. — j. Dubiocarpon sp., Upper Pennsylvanian coal ball, USA, specimen containing one large sphere that in turn contains several smaller spheres. — k, l. Traquairia sp., Lower Pennsylvanian coal ball, Great Britain; k. specimen with preformed aperture from which emerges a fascicle of radially oriented and subdistally constricted protrusions; l. fascicle of protrusions. — m, n. Mycoparasitism in Dubiocarpon sp., Lower Pennsylvanian coal ball, Great Britain; m. specimen containing fungal hypha (arrow); n. spines containing vesicles or propagules (arrows). — o, p. Zygosporites sp., Lower Pennsylvanian coal balls, Great Britain; o. spheroidal specimen; p. ovoid specimen with part of the subtending hypha still in place. — Scale bars: a, f = 200 μm; b, c = 30 μm; d, k, m = 100 μm; e, h, p = 20 μm; g, l, n, o = 50 μm; i = 10 μm; j = 150 μm.
‘Sporocarps’ and other fossils of uncertain affinities
‘Sporocarps’
Within Carboniferous coal balls and chert from Europe and North America are a variety of small (usually < 1 mm diam) spherical structures, including some that are ornamented, which have collectively been termed sporocarps (e.g., Spencer 1893, Hutchinson 1955, Baxter 1960, Davis & Leisman 1962, Stubblefield et al. 1983). However, Krings et al. (2011c) have recently argued that the collective use of the term sporocarp for these fossils may be inaccurate, and thus, if used, be put in quotation marks or inverted commas. ‘Sporocarps’ may occur solitary, but there are many specimens in which several individuals are clustered together (e.g., Williamson 1880, McLean 1922, Hutchinson 1955, Stubblefield et al. 1983). All are composed of a central cavity surrounded by an investment or mantle of loosely arranged interlacing and/or tightly compacted hyphae, which may be aseptate (e.g., Fig. 2g) or septate (e.g., arrow in Fig. 2i). In all ‘sporocarp’ types, there is evidence to suggest that the investment is bounded on the inside by a narrow non-hyphal layer.
Based primarily on investment composition and surface ornamentation, several morphogenera have been introduced for fossil ‘sporocarps’. For example, Mycocarpon (Fig. 2f–i) is characterised by an investment of interlaced hyphae up to four layers thick (Hutchinson 1955, White & Taylor 1991). Specimens of Sporocarpon possess a pseudoparenchymatous investment that extends outward into narrow, conical processes (Stubblefield et al. 1983). A third morphogenus, Dubiocarpon (Fig. 2j, m), is distinguished by an investment constructed of radially elongated segments and spines extending out from the surface (Fig. 2n) (Stubblefield et al. 1983, Gerrienne et al. 1999). The most prominently ornamented taxon is Traquairia (Fig. 2k), a type initially described as a radiolarian rhizopod (Carruthers 1873). The investment of Traquairia is complex, with the outer layer constructed of branching hyphae, some of which are organized into hollow spines (Scott 1911, Stubblefield & Taylor 1983).
While ‘sporocarps’ are relatively common in Pennsylvanian (~318–299 Ma) deposits, they have rarely been reported from geologically older and younger strata. Several forms are known from the Mississippian (~359–318 Ma). One of these, Roannaisia bivitilis, occurs in a Visean (~330 Ma) chert from the Roanne area in France (Taylor et al. 1994), while a second, Mycocarpon cinctum (Fig. 2h, i), comes from Esnost (Rex 1986, Krings et al. 2010), another French locality yielding Visean cherts (Galtier 1971). Two Mississippian representatives of Traquairia have been reported from the vicinity of Burntisland, Scotland (Scott 1911). An interesting ‘sporocarp’ similar to forms known from the Carboniferous is described as Mycocarpon asterineum from the Triassic of Antarctica (Taylor & White 1989). This fossil is characterised by an investment constructed of an outer mycelial and inner noncellular component. In Endochaetophora, a second ‘sporocarp’ type from the Triassic of Antarctica, the investment is tripartite, with the middle layer believed to have formed secondarily between the two pre-existing layers (White & Taylor 1988, 1989a).
‘Sporocarps’ have been generally interpreted as fungal in origin based on the investment constructed of hyphae; the precise systematic affinities of these structures, however, remain elusive. The most controversial aspect concerns the nature of spherical structures present within the central cavity. In some specimens the cavity is empty, but more often contains one to several spheres (e.g., Fig. 2j, k, m) that have been the basis for several hypotheses regarding the affinities of all ‘sporocarps’ in general. One suggests affinities with the Ascomycota based on specimens containing one large sphere believed to represent an ascus that in turn contains several smaller spheres interpreted as ascospores (Stubblefield et al. 1983). According to this idea, the ‘sporocarp’ would represent a cleistothecium. An alternative interpretation views the large sphere as a zygospore, and the entire structure is interpreted as a reproductive structure (i.e. a mantled zygosporangium) of a member of the zygomycetous fungi (Pirozynski 1976, Taylor & White 1989, Krings et al. 2010). If this latter interpretation is accurate, then the smaller spheres found within the large sphere in some specimens (e.g., Fig. 2j) would represent some type of intrusive microfungus.
There is an increasing body of circumstantial evidence to corroborate the latter hypothesis. For example, evidence of mycoparasitism occurs in a specimen of Dubiocarpon from the Lower Pennsylvanian of Great Britain (Krings et al. 2011a). The parasitic fungi are represented by spherical structures (arrows in Fig. 2n), as well as hyphae (arrow in Fig. 2m) forming appressorium-like swellings at the contact region with host walls. This discovery supports the suggestion that many of the small spheres present in other Carboniferous sporocarps may in fact represent stages of mycoparasites. Moreover, a specimen of Traquairia from the Lower Pennsylvanian of Great Britain demonstrates a preformed aperture from which emerges a fascicle of radially oriented structures that are constricted subdistally (arrow in Fig. 2k); transverse septa are present in the constricted areas of some of the structures (Krings et al. 2011c). These outgrowths (Fig. 2l) are morphologically similar to conidiophores bearing terminal conidia of certain extant fungi in the order Entomophthorales, and thus might suggest affinities of Traquairia with the zygomycetous fungi. In addition, the presence of a confluent hyphal meshwork extending around and between clustered specimens has been reported in several representatives of Mycocarpon (e.g., McLean 1922, Stubblefield et al. 1983). This suggests that these structures were produced in groups of two to several, possibly within sporo-carps. Finally, Taylor & White (1989) suggest that the inner, noncellular wall component of the Triassic ‘sporocarp’ Mycocarpon asterineum was produced by a layer of special hyphae along the inner surface of the outer wall layer through continuous secretion of wall material. As the structure expands, the outer wall layer becomes successively compacted. It is interesting that a similar developmental sequence has also been reported in the zoosporangium mantle of the extant zygomycete Endogone flammicorona (Bonfante-Fasolo & Scannerini 1976).
Nevertheless, structural features confirming the zygomycetous affinity of the ‘sporocarps’ have not yet been conclusively documented. Determining the precise affinities of these fossils has been hampered by the fact that virtually all of the specimens discovered to date appear to be at approximately the same stage of development, i.e. fully developed structures. Immature structures would certainly be influenced by preservational bias, and we also believe that they may be rather difficult to accurately identify. A second problem relates to the fact that ‘sporocarps’ always occur isolated or in relatively small clusters, and thus cannot be related to the system on/in which they were produced.
Other enigmatic fossils
There are several other (micro-) fossils in the rock record for which the biological affinities remain unresolved, but that have variously been referred to or compared with zygomycetous fungi. For example, several types of small, ornamented structures occur in abundance in coal balls from the Lower Pennsylvanian of Great Britain (Williamson 1878, 1880, 1883), and also have been discovered in coal balls and chert deposits from elsewhere (Krings et al. 2011b). One of the more common types was named Zygosporites (Williamson 1878, 1880). Specimens of Zygosporites are either spherical (Fig. 2o) or ovoid to elongate (Fig. 2p) and characterised by prominent, antler-like extensions on the exterior surface and a truncated, collar-like extension. Zygosporites was initially believed to represent some type of land plant spore (Williamson 1880) or the zygote of some type of zygnematophycean alga (e.g., Spencer 1893). On the other hand, McLean (1912) noted similarities between Zygosporites and the zygospores of Phycomyces nitens (Mucorales). A recent hypothesis (Krings et al. 2011b), however, suggests that Zygosporites may represent oogonia of peronosporomycetes based on remarkable correspondences of the surface ornamentation patterns to those of the Carboniferous peronosporomycete Combresomyces (see Dotzler et al. 2008, Strullu-Derrien et al. 2011).
Another enigmatic fossil that has been referred to the zygomycetous fungi is Mucor combrensis (Renault 1896), later renamed Mucorites combrensis (Meschinelli 1898), from the upper Visean of France. This fossil occurs in the form of a net-like structure within a lycophyte megaspore (Fig. 3a). However, we believe that the net-like structure interpreted as a mycelium by Renault (1896) simply is a preservational artifact.
Fig. 3.
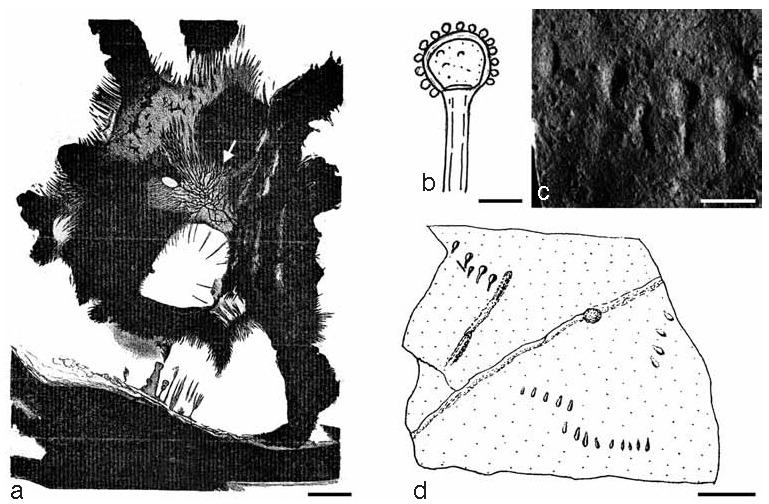
Fossil evidence of zygomycetous fungi (further explanations in the text). a. Mucorites combrensis (arrow), Middle Mississippian chert, France (from Renault 1896: f. 80). — b. Lithomucorites miocenicus, Miocene sediments (dispersed), India (redrawn from Saxena & Tripathi 2011: f. 209). — c, d. Stolophorites lineatus, Triassic shale, USA (from Bock 1969: f. 76, 77); c. part of fossil; d. drawing of specimen. — Scale bars: a, b = 10 μm; c = 5 mm; d = 20 mm.
Lithomucorites miocenicus is an interesting type of dispersed microfossil from the Miocene (~23–5 Ma) of India (Kar et al. 2010, Saxena & Tripathi 2011). It represents what appears to be a fungal sporangium that is apophysate, flask shaped, or (sub)globose, and measures 25–52 × 22–49 μm; some of the specimens occur on the tip of a sporangiophore (Fig. 3b). The wall is closely ornamented with bacula, pila, and verrucae. The fossil has been interpreted as belonging to the zygomycetous fungi because of the presence of coenocytic hyphae, asexual reproduction by means of sporangiophores, and the absence of flagellate cells. Mycozygosporites laevigatus is another dispersed microfossil from the Miocene of India interpreted as belonging to the zygomycetous fungi (Kar et al. 2010). The fossil consists of a thick-walled sphere (believed to represent a zygosporangium) with two tubular hyphae attached opposite to each other.
Amber fossils
Fossil fungi preserved in amber can often be observed and evaluated in great detail by using various microscopy techniques because the translucent nature of the matrix makes it relatively easy to determine even very delicate features useful in systematics and ecology (see Speranza et al. 2010). It is therefore not surprising that fungi in amber were described as early as the nineteenth century (e.g., Goeppert & Berendt 1845). Today there are reports of representatives of many different groups of fungi in amber, including several hyphae and mycelia referred to the zygomycetes (e.g., Grüss 1931, Peñalver et al. 2007, Speranza et al. 2010, Girard & Adl 2011). In addition, Speranza et al. (2010) report on structures in Cenozoic amber from Spain that they believe represent zygospores. None of these records, however, can be regarded as conclusive evidence of zygomycetous fungi.
Poinar & Thomas (1982) describe an entomophthoralean fungus living on the abdomen and thorax of a winged termite preserved in amber from the Dominican Republic (~25 Ma). The body of the animal is covered with a white mat composed of closely appressed, apparently coenocytic hyphae. A layer of conidia lines the surface of the mycelial mat. Some of these conidia are budding and a number of smaller conidia (secondary conidia) were present in the amber just adjacent to the mycelial mat. Based on spore size and shape, these authors conclude that the conidia fall between the ‘Fresenii’ and ‘Lampyridarum’ groups of Entomophthora as defined by Hutchinson (1963) and the ‘Culicis’ group as recognized by Waterhouse (1975). Although the fossil appears rather persuasive at first, we feel that what the authors interpret as conidiophores with terminal conidia might also be a preservational artifact.
Palaeodikaryomyces baueri is a fossil fungus preserved in Cenomanian (Cretaceous; ~99–93 Ma) Schliersee amber (Dörfelt & Schäfer 1998) that is believed to represent a saprotrophic organism occupying a basal position between the Asco- and Basidiomycetes on the one hand and the zygomycetous fungi on the other. The fossil is characterised by aseptate hyphae and vesiculi, developing septa, branches at the vesiculi, clamps or loops, and cysts at the loops (Schönborn et al. 1999). Palaeodikaryomyces baueri is believed to have preserved the essential characters of the primary Dikaryomycetidae, not differentiated into Asco- and Basidiomycetes. Schmidt et al. (2001) hypothesize that P. baueri was an archaic fungus that persisted into the late Mesozoic.
Ichnotaxa
Ichnotaxa (trace fossils) are fossil taxa that are not based on actual organisms, but rather on the fossilized activities of organisms. One ichnotaxon, Stolophorites lineatus from the Upper Triassic (~228–199 Ma) of North America, has been attributed to the zygomycetous fungi (Bock 1969). It consists of several groups of small casts, about 5 mm long, resembling pear or club-shaped forms, i.e. composed of a cone-shaped stalk interpreted as a sporangiophore and terminating in an oval or obtuse head thought to represent a sporangium (Fig. 3c, d). The individual structures are evenly spaced and arranged in straight rows, and appear to be attached to a stolon-like base. Bock (1969) compares the fossils with the extant Rhizopus nigricans. Subsequent workers, however, have regarded the casts as indeterminable (e.g., Olsen & Baird 1990).
CONCLUDING REMARKS
There is an extraordinary abundance of fungal remains in the fossil record. However, systematic studies of fungal lineages based on fossils are lacking to date, due primarily to the inherent problems and limitations connected to the fossil record of the fungi (see above). Moreover, when fungi were reported in the past they were rarely placed within a broader context. During the last twenty years, however, there has been an increasing awareness of fossil fungi and their importance in ancient ecosystems, which has been stimulated by a generally growing scientific interest in the microbial world and the interrelatedness of all organisms today.
Some of the recent discoveries of fossil zygomycetous fungi surveyed in this paper demonstrate that, with suitable preservation, these fungi can be documented in great detail. Such fossils are also of great importance as a source of information that can be used to accurately calibrate molecular clocks and define minimum ages for various fungal lineages. Moreover, it is becoming quite apparent that the fossil record of various lineages of fungi is not only ancient, but also demonstrates a high diversity of forms, some of which closely parallel extant counterparts, even to details relating to micromorphological (cytological) features associated with reproduction. Such comparisons can now be used to discuss the evolution of developmental stages of putatively sexual structures in ancient fungi that heretofore have not been recognised. This will not only increase our understanding about various groups of fungi in time and space, but also when various features evolved. We anticipate that additional and more complete representatives of zygomycetous fungi will be discovered as work on the microbial life preserved in the fossil record continues. This will hopefully lead to a more accurate understanding of the organisms on which the fossils described in this paper were produced. To a large degree we believe that the current underrepresentation of zygomycetous fungi in the fossil record is the result of our inability to recognize the more ephemeral phases and delicate features of these organisms.
On the other hand, enigmatic fossils such as the columella-like structures from the Lower Devonian Rhynie chert and the ‘sporocarps’ from the Carboniferous and Triassic represent interesting components of ancient ecosystems that continue to result in speculation as to their systematic affinities and biological significance. Within these structures there are basic similarities in size and organization that suggest at least some may belong to the same higher taxonomic category, perhaps a lineage of the zygomycetous fungi. Like so many aspects of paleomycology, one specimen often is the single necessary segment of information that helps to elucidate the affinities that have remained elusive. We are certain that this will be the same trajectory regarding all enigmatic fossils detailed in this paper as they are continuously reported and studied.
Acknowledgments
Financial support for part of the research referred to here was provided by the National Science Foundation (EAR-0949947 to TNT and MK) and the Alexander von Humboldt-Foundation (V-3.FLF-DEU/1064359 to MK).
REFERENCES
- Batra LR, Segal RH, Baxter RW.1964. A new Middle Pennsylvanian fossil fungus. American Journal of Botany 51: 991–995 [Google Scholar]
- Baxter RW.1960. Sporocarpon and allied genera from the American Pennsylvanian. Phytomorphology 10: 19–25 [Google Scholar]
- Baxter RW.1975. Fossil fungi from American Pennsylvanian coal balls. University of Kansas Paleontological Contributions 77: 1–6 [Google Scholar]
- Benjamin RK.1979. Zygomycetes and their spores. In: Kendrick B. (ed), The whole fungus. II. The sexual-asexual synthesis. Proceedings of the 2nd International Mycological Conference held at the Environmental Sciences Centre of the University of Calgary Kananaskis, Alberta, Canada: 573–621 National Museum of Natural Sciences, National Museums of Canada, and the Kananaskis Foundation, Ottawa, Canada [Google Scholar]
- Benny GL, Hamber RA, Morton JB.2001. Zygomycota: Zygomycetes. In: McLoughlin DJ, McLoughlin EG, Lemke PE. (eds), The mycota. VIIA. Systematics and evolution: 113–146 Springer-Verlag, Berlin, Germany [Google Scholar]
- Berbee LM, Taylor JW.2001. Fungal molecular evolution: gene trees and geologic time. In: McLaughlin DJ, McLaughlin EG, Lemke PA. (eds), The mycota. VIIB. Systematics and evolution: 229–245 Springer-Verlag, Berlin, Germany [Google Scholar]
- Blair JE.2009. Fungi. In: Hedges SB, Kumar S. (eds), The timetree of life: 215–219 Oxford University Press, New York, NY [Google Scholar]
- Błaszkowski J, Adamska I, Czerniawska B.2004. Endogone lactiflua (Zygomycota, Endogonales) occurs in Poland. Acta Societatis Botanicae Poloniae 73: 65–69 [Google Scholar]
- Błaszkowski J, Tadych M, Madej T.1998. Endogone maritima, a new species in the Endogonales from Poland. Mycological Research 102: 1096–1100 [Google Scholar]
- Bock W.1969. The American Triassic flora and global distribution. Geological Center Research Series 3/4: 1–406 [Google Scholar]
- Bonfante-Fasolo P, Scannerini S.1976. The ultrastructure of the zygospore in Endogone flammicorona Trappe & Gerdemann. Mycopathologia 59: 117–123 [Google Scholar]
- Bucholtz F.1912. Beiträge zur Kenntnis der Gattung Endogone Link. Beihefte zum Botanischen Centralblatt, Abteilung II 29: 147–224 [Google Scholar]
- Butterfield NJ.2005. Probable Proterozoic fungi. Paleobiology 31: 165–182 [Google Scholar]
- Carruthers W.1873. On Traquairia, a radiolarian rhizopod from the coal-measures. Report of the 42nd meeting of the British Association for the Advancement of Science; held at Brighton in August 1872: 126 John Murray, London, UK [Google Scholar]
- Davis B, Leisman GA.1962. Further observations on Sporocarpon and allied genera. Bulletin of the Torrey Botanical Club 89: 97–109 [Google Scholar]
- Degawa Y, Tokumasu S.1997. Zygospore formation in Mortierella capitata. Mycoscience 38: 387–394 [Google Scholar]
- Dilcher DL.1965. Epiphyllous fungi from Eocene deposits in western Tennessee, USA. Palaeontographica 116B: 1–54 [Google Scholar]
- Dörfelt H, Schäfer U.1998. Fossile Pilze im Bernstein der alpischen Trias. Zeitschrift für Mykologie 64: 141–151 [Google Scholar]
- Dotzler N, Krings M, Agerer R, Galtier J, Taylor TN.2008. Combresomyces cornifer gen. sp. nov., an endophytic peronosporomycete in Lepidodendron from the Carboniferous of central France. Mycological Research 112: 1107–1114 [DOI] [PubMed] [Google Scholar]
- Dotzler N, Walker C, Krings M, Hass H, Kerp H, Taylor TN, Agerer R.2009. Acaulosporoid glomeromycotan spores with a germination shield from the 400-million-year-old Rhynie chert. Mycological Progress 8: 9–18 [Google Scholar]
- Fassi B, Fontana A, Trappe JM.1969. Ectomycorrhizae formed by Endogone lactiflua with species of Pinus and Pseudotsuga. Mycologia 61: 412–414 [Google Scholar]
- Galtier J.1971. Sur les flores du Carbonifère inférieur d’Esnost et du Roannais. Bulletin trimestriel de la Société d’histoire naturelle et des amis du Museum d’Autun 57: 24–28 [Google Scholar]
- Gerrienne P, Fairon-Demaret M, Galtier J.1999. A Namurian A (Silesian) permineralized flora from the Carrière du Lion at Engihoul (Belgium). Review of Palaeobotany and Palynology 107: 1–15 [Google Scholar]
- Girard V, Adl SM.2011. Amber microfossils: On the validity of species concept. Comptes Rendus Palevol 10: 189–200 [Google Scholar]
- Goeppert HR, Berendt GC.1845. Der Bernstein und die in ihm befindlichen Pflanzenreste der Vorwelt. Nicolaische Buchhandlung, Berlin, Germany [Google Scholar]
- Grüss J.1931. Die Urform des Anthomyces Reukaufii und andere Einschlüsse in den Bernstein durch Insekten verschleppt. Wochenschrift für Brauerei 48: 63–68 [Google Scholar]
- Hass H, Rowe NP.1999. Thin sections and wafering. In: Jones TP, Rowe NP. (eds), Fossil plants and spores: modern techniques. The Geological Society, London, UK: 76–81 [Google Scholar]
- Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB.2001. Molecular evidence for the early colonization of land by fungi and plants. Science 293: 1129–1133 [DOI] [PubMed] [Google Scholar]
- Hermann TN.1979. Records of fungi from the Riphean. In: Sokolov BS. (ed), Paleontology of Precambrian and Early Cambrian: 129–136 Akademia Nauka SSSR, Leningrad, Russia: [In Russian.] [Google Scholar]
- Hermann TN, Podkovyrov VN.2006. Fungal remains from the Late Riphean. Paleontological Journal 40: 207–214 [Google Scholar]
- Hübers M, Bomfleur B, Krings M, Kerp H.2011. An Early Carboniferous leaf-colonizing fungus. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 261: 77–82 [Google Scholar]
- Hutchinson JA.1963. The genus Entomophthora in the Western Hemisphere. Transactions of the Kansas Academy of Sciences 66: 237–254 [PubMed] [Google Scholar]
- Hutchinson SA.1955. A review of the genus Sporocarpon Williamson. Annals of Botany 19: 425–435 [Google Scholar]
- Javaux EJ, Knoll AH, Walter MR.2001. Morphological and ecological complexity in early eukaryotic ecosystems. Nature 412: 66–69 [DOI] [PubMed] [Google Scholar]
- Kar R, Mandaokar BD, Kar RK.2010. Fungal taxa from the Miocene sediments of Mizoram, northeast India. Review of Palaeobotany and Palynology 158: 240–249 [Google Scholar]
- Karatygin IV, Snigirevskaya NS, Vikulin SV.2009. The most ancient terrestrial lichen Winfrenatia reticulata: a new find and new interpretation. Paleontological Journal 43: 107–114 [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers J.2001. Ainsworth and Bisby’s Dictionary of the Fungi, 9th ed.CAB International, Wallingford, UK [Google Scholar]
- Krings M.2001. Pilzreste auf und in den Fiedern zweier Pteridospermen aus dem Stefan von Blanzy-Montceau (Zentralfrankreich). Abhandlungen des Staatlichen Museums für Mineralogie und Geologie zu Dresden 46/47: 189–196 [Google Scholar]
- Krings M, Dotzler N, Taylor TN.2009. Globicultrix nugax nov. gen. et nov. spec. (Chytridiomycota), an intrusive microfungus in fungal spores from the Rhynie chert. Zitteliana A 48/49: 165–170 [Google Scholar]
- Krings M, Dotzler N, Taylor TN.2011a. Mycoparasitism in Dubiocarpon, a fungal sporocarp from the Carboniferous. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 262: 241–245 [Google Scholar]
- Krings M, Dotzler N, Taylor TN, Galtier J.2010. Microfungi from the upper Visean (Mississippian) of central France: Structure and development of the sporocarp Mycocarpon cinctum nov. sp. Zitteliana A 50: 127–135 [Google Scholar]
- Krings M, Taylor TN.2012a. Fungal reproductive units enveloped in a hyphal mantle from the Lower Pennsylvanian of Great Britain, and their relevance to our understanding of Carboniferous fungal “sporocarps”. Review of Palaeobotany and Palynology 175: 1–9 [Google Scholar]
- Krings M, Taylor TN.2012b. Microfossils with possible affinities to the zygomycetous fungi in a Carboniferous cordaitalean ovule. Zitteliana A 52: 3–7 [Google Scholar]
- Krings M, Taylor TN, Dotzler N.2011b. The fossil record of the Peronosporomycetes (Oomycota). Mycologia 103: 445–457 [DOI] [PubMed] [Google Scholar]
- Krings M, Taylor TN, Dotzler N, Persichini G.2012a. Fossil fungi with suggested affinities to the Endogonaceae from the Middle Triassic of Antarctica. Mycologia 104: 835–844 [DOI] [PubMed] [Google Scholar]
- Krings M, Taylor TN, Taylor EL, Hass H, Kerp H, et al. 2012b. Microfossils from the Lower Devonian Rhynie chert with suggested affinities to the Peronosporomycetes. Journal of Paleontology 86: 358–367 [Google Scholar]
- Krings M, Taylor TN, White JF., Jr2011c. Fungal sporocarps from the Carboniferous: An unusual specimen of Traquairia. Review of Palaeobotany and Palynology 168: 1–6 [Google Scholar]
- Krings M, White JF, Jr, Dotzler N, Harper CJ. In press. A putative zygomycetous fungus with mantled zygosporangia and apposed gametangia from the Lower Coal Measures (Carboniferous) of Great Britain. International Journal of Plant Sciences. [Google Scholar]
- Liu XY, Voigt K.2010. Molecular characters of the zygomycetous fungi. In: Gherbawy Y, Voigt K. (eds), Molecular identification of fungi: 461–488 Springer-Verlag, Berlin, Germany [Google Scholar]
- Liu Y, Steenkamp ET, Brinkmann H, Forget L, Philippe H, Lang BF.2009. Phylogenomic analyses predict sistergroup relationship of nucleariids and fungi and paraphyly of zygomycetes with significant support. BMC Evolutionary Biology 9: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RC.1912. A group of rhizopods from the Carboniferous period. Proceedings of the Cambridge Philosophical Society 16: 493–513 [Google Scholar]
- McLean RC.1922. On the fossil genus Sporocarpon. Annals of Botany 36: 71–90 [Google Scholar]
- Meschinelli A.1898. Fungorum fossilium omnium hucusque cognitorum iconographia 31 tabulis exornata. Typis Aloysii Fabris and C., Venice, Italy [Google Scholar]
- Moczydłowska M, Landing E, Zang W, Palacios T.2011. Proterozoic phytoplankton and timing of chlorophyte algae origins. Palaeontology 54: 721–733 [Google Scholar]
- Nagovitsin K.2009. Tappania-bearing association of the Siberian platform: Biodiversity, stratigraphic position and geochronological constraints. Precambrian Research 173: 137–145 [Google Scholar]
- Olsen PE, Baird D.1990. The ichnogenus Atreipus and its significance for Triassic biostratigraphy. In: Padian K. (ed), The beginning of the age of Dinosaurs. Faunal change across the Triassic-Jurassic boundary: 61–88 Cambridge University Press, Cambridge, New York, NY [Google Scholar]
- Peñalver E, Delclòs X, Soriano C.2007. A new rich amber outcrop with palaeobiological inclusions in the Lower Cretaceous of Spain. Cretaceous Research 28: 791–802 [Google Scholar]
- Petkovits T, Nagy LG, Hoffmann K, Wagner L, Nyilasi I, et al. 2011. Data partitions, bayesian analysis and phylogeny of the zygomycetous fungal family Mortierellaceae, inferred from nuclear ribosomal DNA sequences. PLoS ONE 6, 11: e27507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozynski KA.1976. Fossil fungi. Annual Review of Phytopathology 14: 237–246 [Google Scholar]
- Poinar GO, Thomas GM.1982. An entomophthoralean fungus from Domenican amber. Mycologia 74: 332–334 [Google Scholar]
- Remy W, Taylor TN, Hass H.1994. Early Devonian fungi: a blastocladalean fungus with sexual reproduction. American Journal of Botany 81: 690–702 [Google Scholar]
- Renault B.1896. Bassin Houiller et Permien d’Autun et d’Épinac. Fascicule IV: Flore fossile, deuxième partie. Études des Gîtes Minéraux de la France. Imprimerie Nationale, Paris, France [Google Scholar]
- Rex GM.1986. The preservation and palaeoecology of the Lower Carboniferous silicified plant deposits at Esnost, near Autun, France. Geobios 19: 773–800 [Google Scholar]
- Richardson M.2009. The ecology of the Zygomycetes and its impact on environmental exposure. Clinical Microbiology and Infection 15 (Supplement 5): 2–9 [DOI] [PubMed] [Google Scholar]
- Saxena RK, Tripathi SKM.2011. Indian fossil fungi. Palaeobotanist 60: 1–208 [Google Scholar]
- Schmidt AR, Eynatten H von, Wagreich M.2001. The Mesozoic amber of Schliersee (southern Germany) is Cretaceous in age. Cretaceous Research 22: 423–428 [Google Scholar]
- Schönborn W, Dörfelt H, Foissner W, Krienitz L, Schäfer U.1999. A fossilized microcenosis in Triassic amber. Journal of Eukaryotic Microbiology 46: 571–584 [Google Scholar]
- Scott R.1911. On Traquairia. Annals of Botany 25: 459–467 [Google Scholar]
- Spencer J.1893. Recreations in fossil botany. (Sporocarpons and Zygosporites.) Hardwicke’s science-gossip: An illustrated medium of interchange and gossip for students and lovers of nature 19: 155–158 [Google Scholar]
- Speranza M, Wierzchos J, Alonso J, Bettuchi L, Martín-González A, Ascaso C.2010. Traditional and new microscopy techniques applied to the study of microscopic fungi included in amber. In: Méndez-Vilas A, Díaz J. (eds), Microscopy: science, technology, application and education, vol. 2: 1135–1145 Formatex, Badajoz, Spain [Google Scholar]
- Stanevich AM, Maksimova EN, Kornilova TA, Mazukabzov AM, Gladkochub DP.2007. Microfossils of the Late Proterozoic Debengdinskaya formation of the Olenekskiy uplift. Bulletin of the Tomsk Polytechnic University 311: 9–14 [Google Scholar]
- Strullu-Derrien C, Kenrick P, Rioult JP, Strullu DG.2011. Evidence of parasitic Oomycetes (Peronosporomycetes) infecting the stem cortex of the Carboniferous seed fern Lyginopteris oldhamia. Proceedings of the Royal Society, Series B 278: 675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield SP, Taylor TN.1983. Studies of Paleozoic fungi. I. The structure and organization of Traquairia (Ascomycota). American Journal of Botany 70: 387–399 [Google Scholar]
- Stubblefield SP, Taylor TN, Miller CE, Cole GT.1983. Studies in Carboniferous fungi. II. The structure and organization of Mycocarpon, Sporocarpon, Dubiocarpon, and Coleocarpon (Ascomycotina). American Journal of Botany 70: 1482–1498 [Google Scholar]
- Taylor TN, Galtier J, Axsmith BJ.1994. Fungi from the Lower Carboniferous of central France. Review of Palaeobotany and Palynology 83: 253–260 [Google Scholar]
- Taylor TN, Hass H, Kerp H.1997. A cyanolichen from the Lower Devonian Rhynie chert. American Journal of Botany 84: 992–1004 [PubMed] [Google Scholar]
- Taylor TN, Hass H, Kerp H, Krings M, Hanlin RT.2005a. Perithecial ascomycetes from the 400 million year old Rhynie chert: an example of ancestral polymorphism. Mycologia 97: 269–285 [PubMed] [Google Scholar]
- Taylor TN, Klavins SD, Krings M, Taylor EL, Kerp H, Hass H.2004. Fungi from the Rhynie chert: a view from the dark side. Transactions of the Royal Society of Edinburgh, Earth Sciences 94: 457–473 [Google Scholar]
- Taylor TN, Krings M, Dotzler N, Galtier J.2011. The advantage of thin sections over acetate peels in the study of late Paleozoic fungi and other microorganisms. Palaios 26: 239–244 [Google Scholar]
- Taylor TN, Krings M, Kerp H.2006. Hassiella monospora gen. et sp. nov., a microfungus from the 400 million year old Rhynie chert. Mycological Research 110: 628–632 [DOI] [PubMed] [Google Scholar]
- Taylor TN, Krings M, Klavins SD, Taylor EL.2005b. Protoascon missouriensis, a complex fossil microfungus revisited. Mycologia 97: 725–729 [DOI] [PubMed] [Google Scholar]
- Taylor TN, Remy W, Hass H.1992. Fungi from the Lower Devonian Rhynie chert: Chytridiomycetes. American Journal of Botany 79: 1233–1241 [PubMed] [Google Scholar]
- Taylor TN, Remy W, Hass H, Kerp H.1995. Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87: 560–573 [Google Scholar]
- Taylor TN, Taylor EL, Krings M.2009. Paleobotany. The biology and evolution of fossil plants. Elsevier/Academic Press Inc., Burlington MA, London, San Diego CA, New York NY, USA [Google Scholar]
- Taylor TN, White JF., Jr1989. Fossil fungi (Endogonaceae) from the Triassic of Antarctica. American Journal of Botany 76: 389–396 [Google Scholar]
- Thaxter R.1922. A revision of the Endogoneae. Proceedings of the American Academy of Arts and Sciences 57: 291–351 [Google Scholar]
- Walker C.1985. Endogone lactiflua forming ectomycorrhizas with Pinus contorta. Transactions of the British Mycological Society 84: 353–355 [Google Scholar]
- Waterhouse GM.1975. Key to the species of Entomophthora Fres. Bulletin of the British Mycological Society 9: 15–41 [Google Scholar]
- White MM, James TJ, O’Donnell K, Cafaro MJ, Tanabe Y, Sugiyama J.2006. Phylogeny of the Zygomycota based on nuclear ribosomal sequence data. Mycologia 98: 872–884 [DOI] [PubMed] [Google Scholar]
- White JF, Jr, Taylor TN.1988. Triassic fungus from Antarctica with possible ascomycetous affinities. American Journal of Botany 75: 1495–1500 [Google Scholar]
- White JF, Jr, Taylor TN.1989a. An evaluation of sporocarp structure in the Triassic fungus Endochaetophora. Review of Palaeobotany and Palynology 61: 341–345 [Google Scholar]
- White JF, Jr, Taylor TN.1989b. Triassic fungi with suggested affinities to the Endogonales (Zygomycotina). Review of Palaeobotany and Palynology 61: 53–61 [Google Scholar]
- White JF, Jr, Taylor TN.1991. Fungal sporocarps from Triassic peat deposits in Antarctica. Review of Palaeobotany and Palynology 67: 229–236 [Google Scholar]
- Williamson WC.1878. On the organization of the fossil plants of the coal-measures. Part IX. Philosophical Transactions of the Royal Society of London 169: 319–364 [Google Scholar]
- Williamson WC.1880. On the organization of the fossil plants of the coal-measures. Part X – including an examination of the supposed radiolarians of the Carboniferous rocks. Philosophical Transactions of the Royal Society of London 171: 493–539 [Google Scholar]
- Williamson WC.1883. On the organization of the fossil plants of the coal-measures. Part XII. Philosophical Transactions of the Royal Society of London 174: 459–475 [Google Scholar]
- Yao YJ, Pegler DN, Young TWK.1996. Genera of Endogonales. Royal Botanic Gardens, Surrey, UK [Google Scholar]
- Yin L.1997. Acanthomorphic acritarchs from Meso-Neoproterozoic shales of the Ruyang Group, Shanxi, China. Review of Palaeobotany and Palynology 98: 15–25 [Google Scholar]