Figure 2.
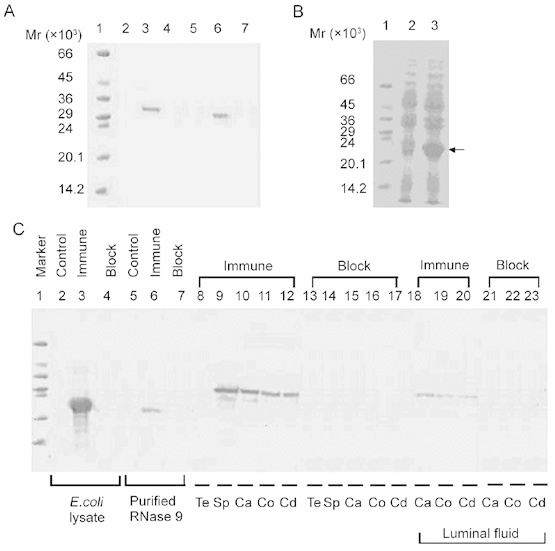
(A): Western blot of mammalian recombinant human RNase 9. Total lysate (lanes 2–4, 10 μg of total protein each lane) and culture supernatant (lanes 5–7, 10 μg of total protein each lane) of HEK293T cells transfected with pcDNA–hRNase9 was probed with normal serum (lanes 2, 5), anti-His antibody (lanes 3, 6) or anti-His antibody that had been preincubated with recombinant RNase 9 (lanes 4, 7). Protein marker on lane 1. (B): Expression of human RNase9 in pET25b (+)-hRNase9 transformed E. coli BL21(DE3). Lane 1, protein marker; lanes 2–3, total cell lysate (50 μg of total protein each lane) of pET25b (+)-hRNase9 transformed E. coli BL21(DE3) without isoprobyl-beta-d-thiogalactopyranoside (IPTG) induction or with IPTG induction for 4 h. (C): Western blot of human RNase 9 antiserum. Total cell lysate protein of pET25b (+)-hRNase9 transformed E. coli (lanes 2–4, 50 μg of total protein each lane), purified recombinant RNase 9 from E. coli (lanes 5–7, 500 ng each lane), and protein extracts from testis (Te), ejaculated sperm (Sp), caput (Ca), corpus (Co), cauda (Cd), epididymal luminal fluid were probed with preimmune serum (control), immune serum or immune serum that had been preincubated with recombinant RNase 9 (block). Equivalent quantities of protein extract (approximately 100 μg) were loaded on lanes 8–23. Protein marker on lane 1.
