Figure 3.
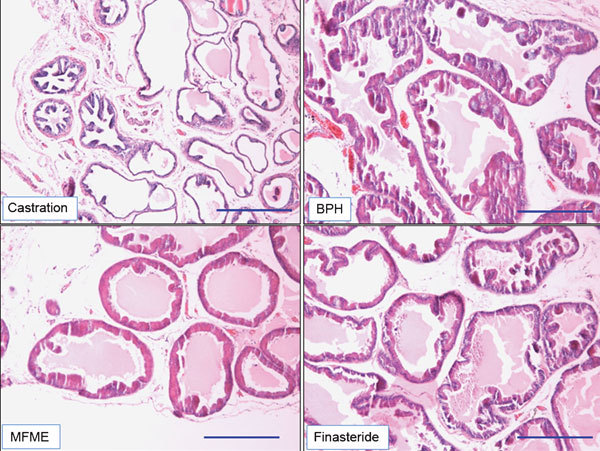
Effects of MFME on prostate hyperplasia. Histological examination of prostate tissue was performed 24 h after the final testosterone injection. Prostate tissues were fixed, sectioned at 4 µm thickness, and stained with H&E solution. Scale bars=49.45 µm. Castration: corn oil injection (s.c.)+oral administration of PBS; BPH: testosterone (s.c.) injection+oral administration of PBS; MFME: oral administration of MFME (200 mg kg−1)+testosterone (s.c.) injection; finasteride: oral administration of finasteride (10 mg kg−1)+testosterone (s.c.) injection. MFME or finasteride treatment was performed 1 h before testosterone injection. BPH, benign prostate hyperplasia; H&E, haematoxylin and eosin; MFME, Melandrium firmum methanolic extract; PBS, phosphate-buffered saline.
