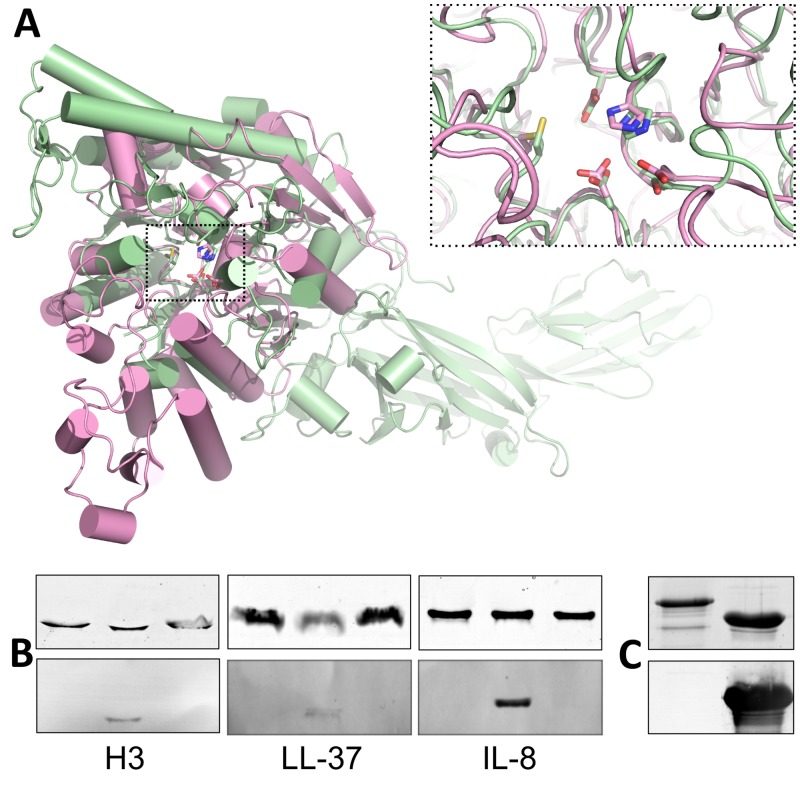
FIG 4 .
Comparison of GAS ADI to human PAD4. (A) Structural alignment of GAS ADI (pink) with human PAD4 (green). The superposition is centered on the α/β-propeller domains, omitting the orthogonal bundle from ADI and the two N-terminal all-β domains from PAD4. ADI active-site residues D166, E220, H275, D277, and C401 align closely with the corresponding residues in PAD4. (B) Unlike human PAD4, GAS ADI does not citrullinate host targets, histone H3, LL-37, or IL-8 in vitro. Host targets were incubated with 5 µM ADI (left lane), 500 nM PAD4 (middle lane), or neither (right lane) for 1 h at 37°C. The reaction was immediately stopped by incubation with SDS-PAGE loading buffer for 15 min at 95°C. Samples containing 1.5 µg of target protein were separated by 15% PAGE and either stained with Coomassie blue (top) or transferred to nitrocellulose membrane for Western blotting with antibodies for the detection of citrulline (bottom). (C) Anti-ADI serum does not recognize recombinant human PAD4 in a Western blot assay. Samples containing 2 µg of ADI (right lane) or PAD4 (left lane) were separated by 15% PAGE and either stained with Coomassie blue (top) or transferred to nitrocellulose membrane for Western blotting (bottom).