Abstract
To date, it remains unclear how herbivore-induced changes in plant primary and secondary metabolites impact above-ground and below-ground herbivore interactions. Here, we report effects of above-ground (adult) and below-ground (larval) feeding by Bikasha collaris on nitrogen and secondary chemicals in shoots and roots of Triadica sebifera to explain reciprocal above-ground and below-ground insect interactions. Plants increased root tannins with below-ground herbivory, but above-ground herbivory prevented this increase and larval survival doubled. Above-ground herbivory elevated root nitrogen, probably contributing to increased larval survival. However, plants increased foliar tannins with above-ground herbivory and below-ground herbivory amplified this increase, and adult survival decreased. As either foliar or root tannins increased, foliar flavonoids decreased, suggesting a trade-off between these chemicals. Together, these results show that plant chemicals mediate contrasting effects of conspecific larval and adult insects, whereas insects may take advantage of plant responses to facilitate their offspring performance, which may influence population dynamics.
Keywords: root, shoot, tannins, flavonoids, Bikasha collaris, Triadica sebifera
1. Introduction
Plants are consumed by above-ground and below-ground herbivores. In the context of above-ground and below-ground compartments, the interactions involved in the plant–herbivore system are complex [1,2]. While above-ground and below-ground herbivores affect plant shoots and/or roots, plants also mediate the interactions between above-ground and below-ground herbivores. Therefore, the outcomes of these complex interactions may vary from positive to neutral or negative, depending on the perspective of component identities (plant or herbivore) and compartments (above-ground or below-ground) [1–6].
Plant chemicals mediate interactions between plants and herbivores [7,8]. For example, tannins are considered especially important in defence against specialist insects as digestibility-reducing compounds [9,10]. Damage by above-ground and/or below-ground herbivores may affect the levels of primary and secondary metabolites in plants, and changes in these chemicals can mediate the interactions of above-ground and below-ground herbivores [11–15]. For instance, Kaplan et al. [11] found in tobacco that root-feeding nematodes increased the performance of folivorous caterpillars by blocking synthesis of alkaloids that are transported to leaves, but caterpillars increased nematode performance by increasing nitrogen transport to roots. In cotton, root-feeding wireworms decreased foliar caterpillar performance by increasing foliar terpenoid levels, but caterpillars had no effect on wireworm performance [16]. In barley, root-feeding wireworms increased foliar aphid reproduction by increasing essential amino acids in leaves, but aphids increased wireworm mass by increasing root mineral content [13]. To date, however, there is no general framework to illustrate how herbivore-induced changes in plant primary and secondary metabolites impact above-ground and below-ground herbivore interactions.
Interactions of above-ground and below-ground herbivores, mediated by plant chemicals, may vary with conditions such as the sequence of herbivory, herbivore type and plant species [2]. Some herbivores, such as flea beetles, have both above-ground (adults) and below-ground (larvae) development stages. Attack in one plant compartment by one development stage can elicit prophylactic defence to subsequent feeding by another development stage in another compartment [5]. Such interactions between parents (adults) and offspring (larvae) may help to genetically link above-ground and below-ground herbivory [17]. Under such conditions, the ‘mother knows best’ hypothesis predicts that there is a strong selection pressure on adults to oviposit on plant parts of high nutrition or low resistance, facilitating offspring survival and development [18]. Because different life stages of the same species (adults versus larvae) may differ in response to direct and indirect defences, studies on a system with above-ground and below-ground life stages of a single species may provide new insights into above-ground and below-ground interactions.
Here, we used Triadica sebifera (tallow tree, Euphorbiaceae; hereafter ‘Triadica’) and its specialist flea beetle, Bikasha collaris (Coleoptera: Chrysomelidae; hereafter ‘Bikasha’) as a study system to document how changes in plant chemicals mediate interactions between above-ground adults and below-ground larvae of a single species. Our previous studies show that Bikasha above-ground feeding adults increased the performance of below-ground feeding conspecific larvae, while larvae negatively impacted adult performance [19]. Furthermore, we found higher adult and larval survival on plants of Triadica populations that have lower tannin contents than on Triadica plants with higher tannin contents [19]. Our recent studies also show that there was a strong negative correlation between larval performance of a specialist caterpillar (Gadirtha inexacta) and tannins content [20,21]. These results led to the hypothesis that levels of chemicals, especially tannins, may affect the interactions of Bikasha above-ground adults and below-ground larvae.
In this study, we extend our previous work with the Triadica–Bikasha system to investigate the effects of above-ground and below-ground herbivory on carbon, nitrogen and secondary chemicals in shoots and roots, and the role of these chemicals in mediating interactions between above-ground and below-ground feeding herbivores. Specifically, we ask: (i) how herbivory by above-ground adults or below-ground larvae, alone or in combination, affect carbon, nitrogen and secondary chemicals in shoots and roots; and (ii) whether such chemical changes are putatively related to reciprocal effects of above-ground and below-ground herbivores.
2. Material and methods
(a). Study organisms
Bikasha is one of the most abundant and damaging specialist insects on Triadica [22,23], a rapidly growing, subtropical tree in southern China [24]. Adults feed on mesophyll tissue, producing irregular pits, whereas larvae burrow into roots and feed internally producing elongate tunnels. Bikasha passes the winter as an adult in soil and debris. It becomes active in late April or early May. Adults lay eggs in the ground, near roots. The embryonic development takes on average 8.7 ± 0.1 days. Larvae take an average of 17.9 ± 0.3 days to reach the pupal stage. The pupal duration averages 8.6 ± 0.2 days and then adults emerge. The generation time of Bikasha is approximately one month [23]. Bikasha is multivoltine, passing through more than five generations per year. Its adult and larval life stages of different generations can be feeding on the same plant concurrently [23].
In late October 2009, we collected seeds of Triadica from a single natural population near Wuhan, China (31°33′ N, 114°07′ E). We germinated seeds following the procedures of Huang et al. [19]. We transplanted seven-week-old seedlings individually into pots (16 cm height, 21 cm diameter) containing growing medium (50% topsoil and 50% sphagnum peat moss) that were arranged in an unheated greenhouse. To reduce potential adverse effects of host-specific soil biota, we collected topsoil from fields where no Triadica grew [25–27]. Subsequently, we enclosed each seedling in a nylon mesh cage (100 cm height, 27 cm diameter) that was fitted tightly to the rim of each pot to exclude insect herbivores. Then all seedlings were allowed to acclimate to ambient conditions for three weeks before the start of experimental treatments.
(b). Experiment design
The experiment was established as a 2 × 2 full factorial design incorporating two levels of above-ground herbivory (0 versus 10 adult Bikasha per plant) and two levels of below-ground herbivory (0 versus 10 larval Bikasha per plant). Each combination was replicated 18 times, yielding a total of 72 plants.
Herbivory treatments followed Huang et al. [19]. In brief, the treatments were timed so that eggs were added for larval treatments at a time such that their hatching (9 days later) would coincide with the addition of adults. Above-ground adults were left on the plants for 18 days. This procedure allowed the periods of above-ground and below-ground herbivory to coincide as the average larval development time is 18 days. To prevent oviposition by adults in the soil and to make below-ground herbivory experimentally independent of above-ground herbivory, the nylon mesh cage of each pot was sealed using string attached to the seedling stem below all leaves. Eggs and adults were from laboratory colonies originally collected from locally established natural populations.
At the end of the herbivory treatment (day 27), we recorded number of surviving adults for each plant (n = 36; 18 with adults only and 18 with adults and larvae). Subsequently, we randomly selected 12 plants of each herbivory combination and harvested them for chemical analysis. We observed and removed adults emerging from the soil on the remaining plants that received larvae (n = 12; six with larvae only and six with adults and larvae) to measure larval to adult survival. This procedure lasted 14 days, sufficient time for pupae to complete development (which typically takes 9 days) [23].
(c). Chemical analyses
Six randomly selected replicates of each herbivory combination were analysed for tannins and flavonoids (n = 24). Leaves, stems and roots were dried at 40°C for 5 days and ground separately. Then samples were assessed by high-performance liquid chromatography, as described by Wang et al. [21]. We measured four tannins (gallic acid, catechin, tannic acid and ellagic acid) and five flavonoids (quercetin, iso-quercetin, quercetin glycoside, kaempferitrin and kaempferol) in leaves, stems and roots.
The remaining six replicates of each herbivory combination were analysed for carbon and nitrogen (n = 24). Leaves, stems and roots were dried at 80°C for 3 days. The ground samples were analysed for total carbon and nitrogen in an elemental autoanalyser (Vario MAX CN; Elementar, Hanau, Germany).
(d). Data analyses
To examine the effect of above-ground adult herbivory (with or without) on below-ground larval survival (arcsin square-root transformed) and the effect of below-ground larvae herbivory (with or without) on above-ground adult survival (arcsin square-root transformed), we performed two separate one-way ANOVAs.
We assessed the effect of above-ground and below-ground herbivory (fixed effects) on total tannins, total flavonoids, carbon and nitrogen in leaves and roots using two-way ANOVAs. To assess the effect of above-ground and below-ground herbivory on levels of individual tannins and flavonoids in leaves and roots, we first conducted two-way MANOVAs (tannins and flavonoids in separate analyses). Then, we used two-way ANOVAs to examine responses of each chemical. We conducted other two-way ANOVAs to examine chemicals in stems. As a complement to these analyses, we performed a pair of three-way repeated ANOVAs to examine effects of above-ground and below-ground herbivory on distribution of tannins and flavonoids (separate analyses) between leaves and roots.
To examine trade-offs between total tannin and flavonoid concentrations, we performed a pair of reduced major axis (RMA) regressions (one for leaves and one for roots). Because there was no clear structure of predictor and response variable, ordinary least square (OLS) regression is not appropriate for this type of analysis [28]. RMA regression does not assume that there is no error in the variable on the x-axis, as does an OLS regression. All data analyses were performed with the statistical software SAS v. 9.0.
3. Results
(a). Herbivore survival
The survival of adults and larvae each depended on the presence of the other life stage (figure 1). The presence of adult herbivores significantly increased larval survival (F1,10 = 12.43, p = 0.006; figure 1a). By contrast, adult survival decreased when larval herbivores were present (F1,34 = 33.06, p < 0.0001; figure 1b).
Figure 1.
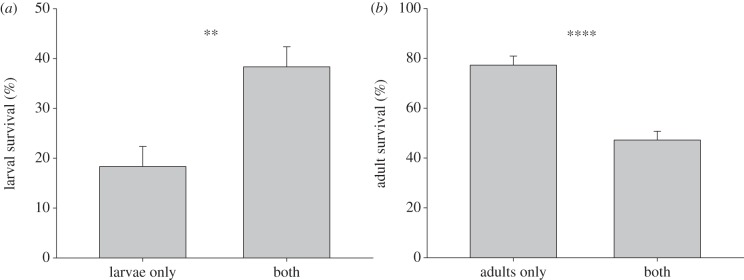
Interactions between adults (above-ground) and larvae (below-ground) of a specialist flea beetle Bikasha collaris via induced Triadica sebifera responses: (a) percentage of larval survival with or without above-ground herbivory, and (b) percentage of adults surviving 18 days with or without below-ground herbivory. Values are means +1 s.e. **p < 0.01; ****p < 0.0001.
(b). Tannins and Flavonoids
Herbivory by adults and larvae each significantly increased foliar tannins, resulting in high concentrations when both types of herbivores were present (table 1 and figure 2a; adults only < both, F1,10 = 6.67, p < 0.05). By contrast, each type of herbivory significantly decreased foliar flavonoids (table 1 and figure 2a). Overall, there was a significant negative relationship between foliar tannins and flavonoids (figure 2a). Root tannins depended significantly on adult herbivory, larval herbivory and their interaction (table 1). Larval herbivory alone resulted in a 4.4-fold increase in root tannins relative to the no-herbivory treatment (table 1 and figure 2b). When plants were exposed to adult herbivores only, root tannins were comparable with the no-herbivory treatment, but with both herbivores present root tannins were significantly lower than when only larval herbivores were present (figure 2b). Root flavonoids depended significantly on adult herbivory and the interaction of adult and larval herbivory (table 1). The adult herbivory only treatment was significantly higher in root flavonoids than no-herbivory and larval herbivory-only treatments, but there were no differences among other pairs of treatment combinations. There was no relationship between root tannins and flavonoids (figure 2b).
Table 1.
Two-way ANOVAs showing the effects of adults (above-ground) and/or larvae (below-ground) of a specialist flea beetle Bikasha collaris on total tannins, total flavonoids, carbon and nitrogen in leaves and roots of Triadica sebifera. Significant results are italicized.
| tissue | compound | d.f. | adults (above-ground) |
larvae (below-ground) |
adults × larvae |
|||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| leaves | total tannins | 1,20 | 73.81 | <0.0001 | 11.80 | 0.0026 | 0.03 | 0.8657 |
| total flavonoids | 1,20 | 14.02 | 0.0013 | 22.60 | 0.0001 | 0.98 | 0.3335 | |
| carbon | 1,20 | 0.31 | 0.5816 | 4.10 | 0.0564 | 16.76 | 0.0006 | |
| nitrogen | 1,20 | 0.18 | 0.6728 | 0.18 | 0.6798 | 1.14 | 0.2992 | |
| roots | total tannins | 1,20 | 271.96 | <0.0001 | 161.25 | <0.0001 | 197.67 | <0.0001 |
| total flavonoids | 1,20 | 6.19 | 0.0218 | 0.35 | 0.5610 | 5.51 | 0.0293 | |
| carbon | 1,20 | 2.02 | 0.1711 | 0.02 | 0.8863 | 0.10 | 0.7532 | |
| nitrogen | 1,20 | 8.94 | 0.0072 | 29.84 | <0.0001 | 0.70 | 0.4124 | |
Figure 2.
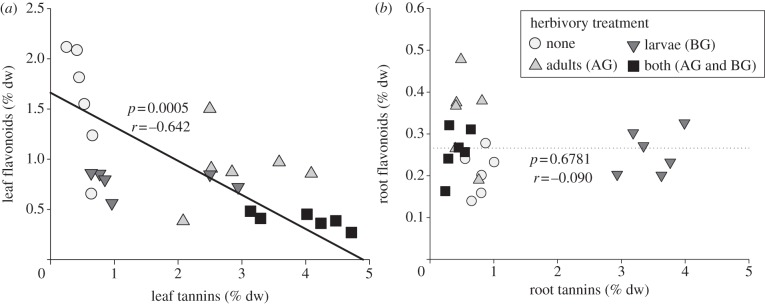
The effects of adults (above-ground, AG) and/or larvae (below-ground, BG) of a specialist flea beetle Bikasha collaris on total tannins and total flavonoids in (a) leaves and (b) roots of Triadica sebifera. Lines, p-values and r-values are from RMA regressions.
On average, every tannin was higher in leaves when adults were present (see electronic supplementary material, table S1 and figure S1), and every foliar flavonoid was highest in the no-herbivory treatment (see electronic supplementary material, table S2 and figure S1). On average, every tannin was highest in roots when only larvae were present, and lowest when both adults and larvae were present (see electronic supplementary material, table S1 and figure S1). Tannins and flavonoids in stems were mostly independent of treatments (see electronic supplementary material, table S3 and figure S1). The phenotypic correlations among roots and shoots depended on the interaction of larvae and adults for tannins, but only varied with larvae and adults as main effects for flavonoids (see electronic supplementary material, table S4).
(c). Carbon and nitrogen
In leaves, carbon depended on the interaction of adult and larval herbivory, but neither factor was significant as a main effect (table 1). When plants were exposed to adult or larval herbivory alone, carbon was reduced relative to the no-herbivory treatment (figure 3). Foliar nitrogen and root carbon were each independent of all treatments (table 1 and figure 3). Herbivory by adults and larvae each significantly increased root nitrogen, resulting in high concentrations when both types of herbivore were present (table 1 and figure 3).
Figure 3.
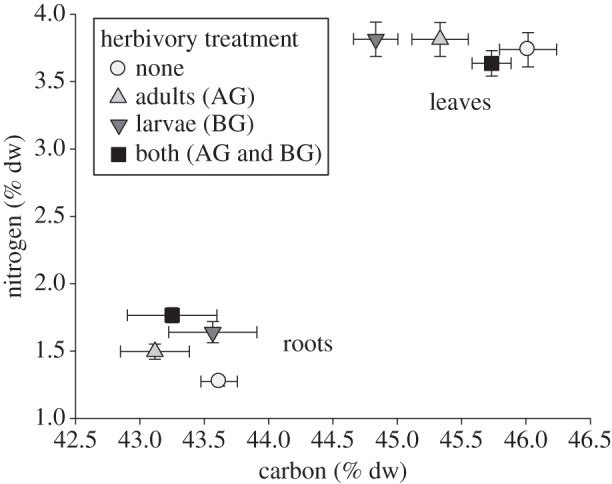
The effects of adults (above-ground, AG) and/or larvae (below-ground, BG) of a specialist flea beetle Bikasha collaris on carbon and nitrogen in leaves and roots of Triadica sebifera. Values are means±1 s.e.
4. Discussion
In this study, we found Bikasha below-ground herbivory increased root tannins in Triadica plants but above-ground herbivory prevented this increase, whereas plants increased foliar tannins with above-ground herbivory and below-ground herbivory amplified this increase. These changes in tannin contents in roots and leaves might be related to the reciprocal effects of above-ground and below-ground herbivores; that is, Bikasha above-ground feeding adults increased the performance of below-ground feeding larvae, whereas larvae negatively impacted adult performance. Additionally, we found above-ground herbivory either alone or in combination with below-ground herbivory elevated root nitrogen, probably contributing to increased larval survival. These results suggest that plants may mediate the contrasting interactive effects of above-ground and below-ground conspecific insect herbivores through changes in nitrogen and secondary metabolites.
The function of tannins in defence against herbivores, specialist insects in particular, has been well known for decades [29]. For example, tannins isolated from Betula resinifera produced approximately linear reductions in growth rate and survival of a beetle (Chrysomela falsa) when doses increased from 0 to 12% [30]. In this study, we found that tannin content in Triadica roots and leaves changed with above-ground adult and below-ground larval feeding, and that the performance of adults and larvae were affected by each other. Our previous study showed that Bikasha adult and larval survivals were higher on plants of Triadica populations that have lower tannin content than those on Triadica plants with higher tannin content [19]. In addition, some of us recently also found that there was a strong negative correlation between larval performance of a specialist caterpillar (G. inexacta) and tannin content in Triadica leaves [20,21]. Taken together, changes in tannins in roots and leaves may contribute to the variable performance of Bikasha above-ground adults and below-ground larvae, and their interactions.
A growing body of evidence indicates that induced defence to above-ground herbivory may be substantially different from that to below-ground herbivory [14,31–33]. The different tannin responses in above-ground and below-ground tissues may also reflect an asymmetric induction and associated transportation in the plant. Tannins are reported to be produced in the leaves of many woody dicots [34], thus, we assume that above-ground herbivory probably only induces and increases tannins in the leaves of Triadica. When roots are attacked, however, tannins may be first synthesized in the leaves and then transported to roots to defend against below-ground herbivory. Our results were consistent with previous findings that above-ground herbivory activated local defence in shoots, and below-ground herbivory elicited systemic defence in both shoots and roots [31,33].
In contrast to the pattern of tannins, flavonoids in the leaves were significantly decreased in all herbivory treatments, suggesting a trade-off between these two classes of defence chemicals. Flavonoids are known to be involved in defence against microbial pathogens, UV and drought, and resistance to nematodes and other generalist herbivore attack [35,36]. Compared with tannins, flavonoids are not as prominent with respect to defence against herbivores, especially to specialists [37]. Thus, regarding defence to herbivory by specialists, such as the flea beetle in this study, tannins may be more important than flavonoids. Furthermore, tannins share a biochemical synthesis pathway with flavonoids. Hydrolyzable tannins (e.g. gallic acid and ellagic acid) are biosynthesized upstream of the flavonoids, and condensed tannins (e.g. catechin) are biosynthesized downstream as polymers of flavonoid molecules [36,38,39]. Thus, when plants are induced by herbivores, increasing hydrolyzable tannins may not correspond to a change in flavonoids, but increasing condensed tannins may result in a decrease in flavonoids.
Primary compounds involved in fundamental plant physiological processes may also have profound effects on the performance of insects [40]. In our study, nitrogen significantly increased in the roots when plants were exposed to above-ground and below-ground herbivores simultaneously. A growing body of evidence shows that most plants contain relatively low amounts of nitrogen, and insect herbivore performance is strongly limited by this nutrient [41]. Thus, our findings that nitrogen increases in the roots following damage by adult, larva and both herbivores may explain the increased below-ground larval performance.
Plant chemicals mediate the interactions of conspecific larval and adult insects, but insects could also take advantage of plant responses to facilitate their offspring performance, which may influence movement patterns and population dynamics. The adult oviposition preference and larval performance hypothesis suggested that adults prefer to oviposit on plants or in habitats where their offspring perform well [18]. Our results suggest that feeding by above-ground adults may itself increase the suitability of a host plant for below-ground larvae through decreasing tannin content and increasing nitrogen in roots. Indeed, the benefits of ovipositing on a plant receiving above-ground damage appear to be substantial. Conversely, the reduced performance of adults on plants with below-ground feeding larvae suggests benefits to adults dispersing to plants without root-feeding larvae following emergence. However, it is not known whether adult flea beetles recognize the presence of larvae or tend to choose plants for feeding or oviposition based on the presence of different herbivore life stages [2,5].
Herbivores with above-ground and below-ground life stages that feed on the same host plant are common in nature, including many Coleopteran species in the Chrysomelidae, Curculionidae and Scarabaeidae families. Study of the interactions of above-ground and below-ground life stages may provide new insights into the understanding of how those species interact with other heterospecific insects to shape communities. In addition to Bikasha, Triadica also supports many other above-ground herbivores, including caterpillars, weevils and leaf beetles [22]. The interactions of those above-ground herbivores with Bikasha larvae may be different from those for Bikasha adults. We expect that heterospecific defoliators may compete with Bikasha adults and inhibit Bikasha larvae as a result of resource competition. Also, relative to conspecific above-ground and below-ground insects that have genetic links (such as Bikasha), herbivore-induced defences in plant shoots and/or roots may differ from that of heterospecific insects [5,17]. Further studies may help to better understand above-ground and below-ground insect community structure, and unravel the mechanisms underlying the complex interactions among heterospecific insects and species having both above-ground and below-ground life stages.
To understand above-ground–below-ground interactions under natural conditions, experiments should be conducted using the natural temporal sequences of above-ground and below-ground herbivores. For systems with differing above-ground and below-ground herbivore species, their interactions may be affected by the sequence of herbivory, herbivore types and host plant species [2]. For instance, Erb et al. [42] found in maize that noctuid moth feeding had a significant negative effect on colonization by a root-feeding beetle, but only when above-ground herbivores attacked the plant first. Most previous studies used different above-ground and below-ground herbivore species, while tests on herbivore species that have both above-ground and below-ground life stages are rare [43]. Specialist flea beetles and root weevils whose adults eat leaves and larvae attack roots, however, may provide important systems for study of above-ground–below-ground interactions. As many of them, such as Bikasha in our study system, are multivoltine, the adult and larval life stages of different generations can be feeding on the same plant at the same time in nature, providing an ecological realism for using above-ground and below-ground herbivore combinations. A Bikasha adult can live as long as 50 days, whereas its larval stage is usually only 15–20 days. As the beetle can pass through more than five generations per year, herbivory by adults and larvae often overlap.
Studies on a system with above-ground and below-ground life stages of a single species may also have important applied implications. Flea beetles and root weevils are often destructive agricultural pests or successful biological control agents of invasive plants. They have been recorded as important pests on soya beans, sorghum, grain, corn and several vegetable crop species. For example, corn flea beetles (Chaetocnema pulicaria) may cause severe damage on sweetcorn crops, and also carry Stewart's wilt bacterial disease, which may result in 40–100% yield loss [44]. With regard to biological control of invasive plants, Bikasha is being considered as a biological control agent for Triadica, which has become an important invasive plant in southeastern United States [23]. In addition, as biological control agents, the flea beetles Aphthona nigriscutis and Aphthona lacertosa were successful in the management of invasive weed leafy spurge (Euphorbia esula) in North Dakota by increasing native species richness and decreasing leafy spurge density by an average of 94% [45]. Thus, understanding above-ground and below-ground herbivore interactions of such species may help to improve pest management or weed biological control.
In summary, our study suggests that the contrasting interactive effects of above-ground and below-ground conspecific insect herbivores may be mediated by changes in plant nitrogen and secondary chemicals. While plants produce chemicals to respond to above-ground and below-ground herbivory, insects may take advantage of the responses to facilitate their offspring performance and impact population dynamics. These findings help us to better understand how plants evolve with above-ground and below-ground herbivores, and how these herbivores interact through plant chemical changes.
Acknowledgements
We would like to thank Juli Carrillo and Yi Wang for comments and discussions, and Lin Zhu, Xue Gu and Wei Hui for field assistance. We are grateful to two anonymous reviewers for useful comments on the earlier versions of this paper.
Funding statement
This study was supported by the China National Basic Study Program (2012CB114104 to J.D.), the National Natural Science Foundation of China (31200286 to W.H.), the US National Science Foundation (DEB 0820560 to E.S.), the Florida Fish and Wildlife Commission and Florida Department of Environmental Protection (SL849 to G.S.W.), and the Foreign Visiting Professorship of the Chinese Academy of Sciences (to E.S.).
References
- 1.Soler R, Erb M, Kaplan I. 2013. Long distance root-shoot signalling in plant–insect community interactions. Trends Plant Sci. 18, 149–156 (doi:10.1016/j.tplants.2012.08.010) [DOI] [PubMed] [Google Scholar]
- 2.Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW. 2012. Aboveground–belowground herbivore interactions: a meta-analysis. Ecology 93, 2208–2215 (doi:10.1890/11-2272.1) [DOI] [PubMed] [Google Scholar]
- 3.Bezemer TM, van Dam NM. 2005. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 20, 617–624 (doi:10.1016/j.tree.2005.08.006) [DOI] [PubMed] [Google Scholar]
- 4.Erb M, Ton J, Degenhardt J, Turlings TCJ. 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol. 146, 867–874 (doi:10.1104/pp.107.112169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dam NM, Heil M. 2011. Multitrophic interactions below and above ground: en route to the next level. J. Ecol. 99, 77–88 (doi:10.1111/j.1365-2745.2010.01761.x) [Google Scholar]
- 6.Wondafrash M, van Dam NM, Tytgat TOG. 2013. Plant systemic induced responses mediate interactions between root parasitic nematodes and aboveground herbivorous insects. Front. Plant Sci. 4, 87 (doi:10.3389/fpls.2013.00087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bednarek P. 2012. Chemical warfare or modulators of defence responses: the function of secondary metabolites in plant immunity. Curr. Opin. Plant Biol. 15, 407–414 (doi:10.1016/j.pbi.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 8.Mithöfer A, Boland W. 2012. Plant defense against herbivores: chemical aspects. Annu. Rev. Plant Biol. 63, 431–450 (doi:10.1146/annurev-arplant-042110-103854) [DOI] [PubMed] [Google Scholar]
- 9.Forkner RE, Marquis RJ, Lill JT. 2004. Feeny revisited: condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecol. Entomol. 29, 174–187 (doi:10.1111/j.1365-2311.2004.0590.x) [Google Scholar]
- 10.Müller-Schärer H, Schaffner U, Steinger T. 2004. Evolution in invasive plants: implications for biological control. Trends Ecol. Evol. 19, 417–422 (doi:10.1016/j.tree.2004.05.010) [DOI] [PubMed] [Google Scholar]
- 11.Kaplan I, Halitschke R, Kessler A, Rehill BJ, Sardanelli S, Denno RF. 2008. Physiological integration of roots and shoots in plant defense strategies links above- and belowground herbivory. Ecol. Lett. 11, 841–851 (doi:10.1111/j.1461-0248.2008.01200.x) [DOI] [PubMed] [Google Scholar]
- 12.Erb M, Flors V, Karlen D, De Lange E, Planchamp C, D'Alessandro M, Turlings TCJ, Ton J. 2009. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J. 59, 292–302 (doi:10.1111/j.1365-313X.2009.03868.x) [DOI] [PubMed] [Google Scholar]
- 13.Johnson SN, Hawes C, Karley AJ. 2009. Reappraising the role of plant nutrients as mediators of interactions between root- and foliar-feeding insects. Funct. Ecol. 23, 699–706 (doi:10.1111/j.1365-2435.2009.01550.x) [Google Scholar]
- 14.Rasmann S, Agrawal AA, Cook SC, Erwin AC. 2009. Cardenolides, induced responses, and interactions between above- and belowground herbivores of milkweed (Asclepias spp.). Ecology 90, 2393–2404 (doi:10.1890/08-1895.1) [DOI] [PubMed] [Google Scholar]
- 15.Erb M, Köllner TG, Degenhardt J, Zwahlen C, Hibbard BE, Turlings TCJ. 2011. The role of abscisic acid and water stress in root herbivore-induced leaf resistance. New Phytol. 189, 308–320 (doi:10.1111/j.1469-8137.2010.03450.x) [DOI] [PubMed] [Google Scholar]
- 16.Bezemer TM, Wagenaar R, van Dam NM, Wäckers FL. 2003. Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562 (doi:10.1034/j.1600-0706.2003.12424.x) [Google Scholar]
- 17.Johnson SN, Birch ANE, Gregory PJ, Murray PJ. 2006. The ‘mother knows best’ principle: should soil insects be included in the preference-performance debate? Ecol. Entomol. 31, 395–401 (doi:10.1111/j.1365-2311.2006.00776.x) [Google Scholar]
- 18.Valladares G, Lawton JH. 1991. Host-plant selection in the holly leaf-miner: does mother know best? J. Anim. Ecol. 60, 227–240 (doi:10.2307/5456) [Google Scholar]
- 19.Huang W, Carrillo J, Ding J, Siemann E. 2012. Invader partitions ecological and evolutionary responses to above- and belowground herbivory. Ecology 93, 2343–2352 (doi:10.1890/11-1964.1) [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Siemann E, Wheeler GS, Zou J, Carrillo J, Ding J. 2010. Resource allocation to defence and growth are driven by different responses to generalist and specialist herbivory in an invasive plant. J. Ecol. 98, 1157–1167 (doi:10.1111/j.1365-2745.2010.01704.x) [Google Scholar]
- 21.Wang Y, Siemann E, Wheeler GS, Zhu L, Gu X, Ding J. 2012. Genetic variation in anti-herbivore chemical defences in an invasive plant. J. Ecol. 100, 894–904 (doi:10.1111/j.1365-2745.2012.01980.x) [Google Scholar]
- 22.Zheng H, Wu Y, Ding J, Binion D, Fu W, Reardon R. 2005. Invasive plants established in the United States that are found in Asia and their associated natural enemies. Morgantown, WV: Forest Health Technology Enterprise Team [Google Scholar]
- 23.Huang W, Wheeler GS, Purcell MF, Ding J. 2011. The host range and impact of Bikasha collaris (Coleoptera: Chrysomelidae), a promising candidate agent for biological control of Chinese tallow, Triadica sebifera (Euphorbiaceae) in the United States. Biol. Control 56, 230–238 (doi:10.1016/j.biocontrol.2010.11.014) [Google Scholar]
- 24.Zhang KD, Lin YT. 1994. Chinese tallow. Beijing, China: China Forestry Press [Google Scholar]
- 25.Bezemer TM, De Deyn GB, Bossinga TM, van Dam NM, Harvey JA, van der Putten WH. 2005. Soil community composition drives aboveground plant–herbivore–parasitoid interactions. Ecol. Lett. 8, 652–661 (doi:10.1111/j.1461-0248.2005.00762.x) [Google Scholar]
- 26.Monroy F, van der Putten WH. 2009. Local variation in belowground multitrophic interactions. Soil Biol. Biochem. 41, 1689–1695 (doi:10.1016/j.soilbio.2009.05.011) [Google Scholar]
- 27.van der Putten WH, Kowalchuk GA, Brinkman EP, Doodeman GTA, van der Kaaij RM, Kamp AFD, Menting FBJ, Veenendaal EM. 2007. Soil feedback of exotic savanna grass relates to pathogen absence and mycorrhizal selectivity. Ecology 88, 978–988 (doi:10.1890/06-1051) [DOI] [PubMed] [Google Scholar]
- 28.Sokal RR, Rohlf FJ. 1994. Biometry: the principles and practices of statistics in biological research, 3rd edn New York: WH Freeman and Company [Google Scholar]
- 29.Feeny P. 1976. Plant apparency and chemical defense. Recent Adv. Phytochem. 10, 1–40 (doi:10.1007/978-1-4684-2646-5_1) [Google Scholar]
- 30.Ayres MP, Clausen TP, MacLean SF, Redman AM, Reichardt PB. 1997. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 78, 1696–1712 (doi:10.1890/0012-9658(1997)078[1696:dosaaa]2.0.co;2) [Google Scholar]
- 31.Bezemer TM, Wagenaar R, van Dam NM, van Der Putten WH, Wackers FL. 2004. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J. Chem. Ecol. 30, 53–67 (doi:10.1023/B:JOEC.0000013182.50662.2a) [DOI] [PubMed] [Google Scholar]
- 32.Rasmann S, Turlings TCJ. 2007. Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol. Lett. 10, 926–936 (doi:10.1111/j.1461-0248.2007.01084.x) [DOI] [PubMed] [Google Scholar]
- 33.Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF. 2008. Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89, 392–406 (doi:10.1890/07-0471.1) [DOI] [PubMed] [Google Scholar]
- 34.Barbehenn RV, Constabel PC. 2011. Tannins in plant–herbivore interactions. Phytochemistry 72, 1551–1565 (doi:10.1016/j.phytochem.2011.01.040) [DOI] [PubMed] [Google Scholar]
- 35.Treutter D. 2006. Significance of flavonoids in plant resistance: a review. Environ. Chem. Lett. 4, 147–157 (doi:10.1007/s10311-006-0068-8) [Google Scholar]
- 36.Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V. 2011. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 62, 2465–2483 (doi:10.1093/jxb/erq442) [DOI] [PubMed] [Google Scholar]
- 37.Bernards MA, Bastrup-Spohr L. 2008. Phenylpropanoid metabolism induced by wounding and insect herbivory. In Induced plant resistance to herbivory (ed. Schaller A.), pp. 189–213 New York, NY: Springer [Google Scholar]
- 38.Dixon RA, Xie D-Y, Sharma SB. 2005. Proanthocyanidins: a final frontier in flavonoid research? New Phytol. 165, 9–28 (doi:10.1111/j.1469-8137.2004.01217.x) [DOI] [PubMed] [Google Scholar]
- 39.Vogt T. 2010. Phenylpropanoid biosynthesis. Mol. Plant 3, 2–20 (doi:10.1093/mp/ssp106) [DOI] [PubMed] [Google Scholar]
- 40.Berenbaum MR. 1995. Turnabout is fair play: secondary roles for primary compounds. J. Chem. Ecol. 21, 925–940 (doi:10.1007/BF02033799) [DOI] [PubMed] [Google Scholar]
- 41.Awmack CS, Leather SR. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844 (doi:10.1146/annurev.ento.47.091201.145300) [DOI] [PubMed] [Google Scholar]
- 42.Erb M, Robert CAM, Hibbard BE, Turlings TCJ. 2011. Sequence of arrival determines plant-mediated interactions between herbivores. J. Ecol. 99, 7–15 (doi:10.1111/j.1365-2745.2010.01757.x) [Google Scholar]
- 43.Clark KE, Hartley SE, Johnson SN. 2011. Does mother know best? The preference–performance hypothesis and parent–offspring conflict in aboveground–belowground herbivore life cycles. Ecol. Entomol. 36, 117–124 (doi:10.1111/j.1365-2311.2010.01248.x) [Google Scholar]
- 44.Pataky JK. 2004. Stewart's wilt of corn. See http://www.apsnet.org/edcenter/intropp/lessons/prokaryotes/Pages/StewartWilt.aspx (doi:10.1094/PHI-I-2004-0113-01) [Google Scholar]
- 45.Setter CM, Lym RG. 2013. Change in leafy spurge (Euphorbia esula) density and soil seedbank composition 10 years following release of Aphthona spp. biological control agents. Invasive Plant Sci. Manag. 6, 147–160 (doi:10.1614/ipsm-d-12-00031.1) [Google Scholar]


