Abstract
Globozoospermia is a severe form of teratozoospermia characterized by round-headed spermatozoa with an absent acrosome, an aberrant nuclear membrane and midpiece defects. Globozoospermia is diagnosed by the presence of 100% round-headed spermatozoa on semen analysis, and patients with this condition are absolutely infertile. The objective of this study was to investigate the differences in protein expression between human round-headed and normal spermatozoa. Two-dimensional (2-D) fluorescence difference gel electrophoresis (DIGE) coupled with mass spectrometry (MS) was used in this study. Over 61 protein spots were analysed in each paired normal/round-headed comparison, using DIGE technology along with an internal standard. In total, 35 protein spots identified by tandem mass spectrometry (MS/MS) exhibited significant changes (paired t-test, P < 0.05) in the expression level between normal and round-headed spermatozoa. A total of nine proteins were found to be upregulated and 26 proteins were found to be downregulated in round-headed spermatozoa compared with normal spermatozoa. The differentially expressed proteins that we identified may have important roles in a variety of cellular processes and structures, including spermatogenesis, cell skeleton, metabolism and spermatozoa motility.
Keywords: differential protein, globozoospermia, mass spectrometry (MS), two-dimensional difference gel electrophoresis (2-D DIGE)
Introduction
Globozoospermia is a severe form of teratozoospermia characterized by round-headed spermatozoa with an absent acrosome, an aberrant nuclear membrane and midpiece defects. This alteration was shown by analysing a population of infertile men who were all part of the same family and had a history of consanguinity. No abnormalities on the physical examination of affected males or peculiarities in the case history have previously been associated with globozoospermia. Total globozoospermia is diagnosed by the presence of 100% round-headed spermatozoa on semen analysis and patients with this condition are always infertile. The introduction of intracytoplasmic spermatozoa injection (ICSI) technology has provided a fertility treatment for patients suffering from certain spermatozoa defects, such as globozoospermia. Pregnancy with globozoospermic, acrosomeless spermatozoa delivered by ICSI was first reported in 1994 1. Since then, more successful attempts at ICSI with globozoospermic spermatozoa have been reported 2, 3, 4, 5. However, both fertilization and pregnancy rates are significantly lower in ICSI performed with globozoospermic spermatozoa than are observed with ICSI in general 6, 7. Furthermore, abnormal spermatozoa morphology might be associated with genetic alterations in the spermatozoa, which could have adverse consequences on patients' offspring and future generations. Since the first reported globozoospermia case was described, more descriptions of morphological and aetiological abnormalities have been detected in globozoospermic spermatozoa 8. In some cases, an increased number of spermatozoa cells with fragmented DNA have been observed in globozoospermic patients 9, 10. Recent studies have shown that globozoospermia is associated with cytogenetic abnormalities such as chromosomal aneuploidies 11. Rubes et al. 12 found that the number of round-headed spermatozoa cells is significantly increased in the ejaculate of smokers. In addition, specific Y-chromosome microdeletions have been found in patients with globozoospermia. Although several hypotheses, including autosomal dominant, autosomal recessive, monogenic and polygenic gene mutations, have been suggested as possible causes of globozoospermia, the specific aetiology of globozoospermia is unclear. A genetic component seems to be part of the picture, because this rare syndrome has been identified in multiple members of the same family on several occasions 13. Patients with globozoospermia showed a wide spectrum of abnormal semen characteristics. Thus, it is probable that multiple genetic components contribute to the disorder. Few genetic lesions have been linked to globozoospermia and most of them affect germ cell acrosome biogenesis. Several candidates, including those originally believed to be involved in the regulation of the spermatozoa head morphology, have been identified as genes specific for male infertility. The Csnk2a2 14, HIV-1 Rev-binding protein (Hrb) 15 and Golgi-associated PDZ and coiled–coil motif-containing protein (GOPC) 16, are of particular interest, because the phenotypes of Csnk2a2, Hrb and COPC-null mice are very similar to those of humans with globozoospermia. In addition to being infertile, the spermatozoa produced by these three knockout mice are primarily round-headed and lack acrosomes. However, genetic research on globozoospermia is still in its infancy. In 2004, Luo et al. 17 acquired 16 spermatozol spots that were differentially expressed in normal and round-headed spermatozoa. The authors identified eight of them, but the identified proteins were quite limited because of technological limitation, thus, further research was needed to strengthen this study.
Proteins are the direct executants of cells and organisms. To know the exact proteome of round-headed spermatozoa and how it differs from that of normal spermatozoa would not only help to investigate round-headed spermatogenesis and its underlying pathophysiology but also assist further research on the identification of the aetiological factors underlying male infertility. Thus, it is of great research interest to identify a group of proteins with consistently changing expression levels, whose function may reveal insight into the critical events that occur during the disease progression of globozoospermia, as they may be valuable in terms of identifying potential therapeutic targets. In this study, we performed a comparative analysis of spermatozoa proteins found in normal and round-headed spermatozoa using the novel two-dimensional (2-D) fluorescence difference gel electrophoresis (DIGE) technique. We identified a group of proteins that were up- and downregulated in round-headed spermatozoa as compared with normal spermatozoa, and subsequent bioinformatic analyses indicated that these proteins may have important roles in a variety of cellular processes and structures, including spermatogenesis, cell signalling, cell skeleton and metabolism.
Materials and methods
Materials
Cy2, Cy3, Cy5, immobilized pH gradient (IPG) strips and IPG buffer were purchased from GE Healthcare (Little Chalfont, Bucks, UK). Thiourea was purchased from Fluka (Buchs, Switzerland). Urea, CHAPS (3-[(3-cholanidopropyl)dimethylammonio]-1-propanesulfonate), DTT (1, 4-dithio-DL-threitol) and the Bradford assay kit were purchased from BioRad (Bio-Rad Laboratories, Hercules, CA, USA), and the complete protease inhibitor cocktail tablet was purchased from Roche (Mannheim, Germany). Modified trypsin (sequencing grade) was obtained from Promega (Madison, WI, USA). All other chemicals and biochemical reagents used were of analytical grade.
Collection of spermatozoa samples
All samples used in this research were obtained with informed consent from every donor and with the approval of the National Human Reproduction Ethic Investigation Committee. The round-headed spermatozoa samples were obtained from a patient who has exclusively round-headed acrosomeless spermatozoa cells, as determined by light and electron microscope. The globozoospermia spermatozoa used in this study were obtained from 20 ejaculates from one patient with this syndrome. Normal spermatozoa samples were obtained from 12 fertile donors with proven fertility. Normal spermatozoa samples were obtained by masturbation after 4–6 days of abstinence. Each donor donated three separate samples.
Spermatozoa isolation
Spermatozoa cells were isolated from the semen using a 1-step Percoll gradient (75%) and performing centrifugation at 600 × g for 30 min. Spermatozoa with potentially contaminated cells were recovered from the 75% interface. The spermatozoa pellet was collected and washed with phosphate-buffered saline (PBS) thrice by centrifugation at 600 × g for 10 min at room temperature. After the last centrifugation, the spermatozoa were pooled, frozen immediately and stored in liquid nitrogen until use.
Solubilization of spermatozoaatozoa
Spermatozoa were routinely lysed in lysis buffer (7 mol L−1 urea, 2 mol L−1 thiourea, 4% CHAPS, 10 mmol L−1 Tris and 5 mmol L−1 magnesium acetate) with one complete protease inhibitor cocktail tablet added per 50 mL of lysis buffer 18. After centrifugation at 12 000 × g for 30 min at 4°C, all non-solubilized material was removed. The protein concentration was determined with a Bradford assay kit (BioRad) using albumin diluted in lysis buffer as the reference standard. The supernatant was used for 1-D electrophoresis either immediately after preparation or stored at −80°C for future use.
Protein labelling with Cy-Dye DIGE fluor
Samples were labelled with three Cy-Dye DIGE fluors, Cy2, Cy3 and Cy5. This process was carried out according to the Ettan DIGE user's manual (18-1164-40 Edition AA, GE Healthcare). A total of 50 μg of protein sample was labelled with 400 pmol of Cy3 or Cy5. An internal reference standard, consisting of two mixed samples used in the experiment, was labelled with Cy2. The labelling reaction was conducted on ice for 30 min under dark conditions. Reactions were quenched by the addition of 10 mmol L−1 lysine and incubated for 10 min on ice under dark conditions. The labelled samples were pooled and prepared for the subsequent steps in the experiment. To demonstrate the reproducibility and reliability of our results, each sample was separated twice on separate gels.
Protein separation by 2-D fluorescence DIGE
Two-D electrophoresis was performed as described earlier with several minor modifications 19. IPG strips (24 cm, PH3-10 and NL) were hydrated in hydration buffer (8 mol L−1 urea, 4% CHAPS, 20 mmol L−1 DTT, 1% v/v IPG buffer and a few grains of bromphenol blue) and incubated with labelled samples under dark conditions. The first-dimension IEF was performed using an Ettan IPGphor System (GE Healthcare) for a total of 65 kV h−1 at 20°C. After the IEF, the IPG strips were equilibrated in the equilibration solution (6 mol L−1 urea, 30% glycerol, 2% sodium dodecyl sulphate and 50 mmol L−1 of Tris-Cl, PH 8.8) containing 1% w/v DTT for 15 min and then in the same solution containing 3% IAA instead of DTT. Second-dimension separation was performed at 2 W per gel at 1°C (Ettan DALT12 system, GE Healthcare) using hand-cast 12.5% gels. Voltages and currents were continuously monitored throughout all runs for quality control.
Scanning of DIGE-labelled images
Labelled proteins were visualized using the Typhoon 9410 imager (Amersham Biosciences). The Cy5 images were scanned using a 633-nm laser and a 670-nm band pass (BP) 30 emission filter. Cy3 images were scanned using a 532-nm laser and a 580-nm BP30 emission filter. Cy2 images were scanned using a 488-nm laser and a 520-nm BP40 emission filter. The narrow BP emission filters ensured that there was negligible crosstalk between fluorescence channels. All gels were scanned at 100 μm resolution.
Image analysis
The scanned fluorescent gel images Cy-Dyes were analysed using DeCyder Differential Analysis Software (Version 5.02, GE Healthcare/Amersham Biosciences). DeCyder is an image analysis program for spot detection, matching and quantitation of images generated using the Ettan DIGE system. Differential in-gel analysis (DIA) was used to calculate protein abundance variations between samples on the same gel. The resulting spot maps were then analysed through biological variation analysis to provide statistical data on the differential protein expression that existed between the normal and round-headed groups. The Cy3:Cy2 and Cy5:Cy2 DIA ratios were used to calculate average protein abundance changes, and paired t-test P-values for the variances of these ratios were calculated for each protein pair across all samples. The benefit of the internal reference standard is that it increases the investigator's confidence in results obtained from different gels.
Spot picking and enzymatic digestion
Separate preparative gels were run to obtain sufficient amounts of protein for mass spectrometry (MS) analysis. The gels were fixed and stained with colloidal coomasie brilliant blue (cCCB) 20. Proteins of interest (those with a greater than twofold difference in spot volume ratio, P < 0.05), as defined by 2-D DIGE/DeCyder analysis, were excised from the cCBB-stained gels manually for modified in-gel tryptic digestion procedure. In brief, gel pieces were first destained with 50% acetonitrile (ACN) and 25 mmol L−1 of ammonium bicarbonate, then all destained spots were dried in a vacuum pump, reduced by 10 mmol L−1 DTT (composed of 50 mmol L−1 NH4HCO3) for 30 min at 56°C, followed by replacement of DTT with 55 mmol L−1 IAA solution (made up with 50 mmol L−1 ammonium bicarbonate) and alkylation for 30 min under dark conditions. Then, the spots were dehydrated in 100 mmol L−1 NH4HCO3, digested using trypsin digestion work solution (T 6 567, Sigma) and incubated for 15–18 h. Supernatants were collected, vacuum-dried and dissolved in 50% ACN and 0.1% trifluoroacetic acid (TFA) for MS analysis.
Matrix-assisted laser desorption/ionization-Time of Flight/Time of Flight analysis
Peptides were mixed with MALDI matrix (7 mg mL−1 CHCA (α-cyano-4-hydroxycinnamic acid), 0.1% TFA and 50% ACN) and spotted onto 192-well stainless steel MALDI-target plates. Samples were then analysed using an ABI 4600 Proteomics Analyzer MALDI-TOF/TOF mass spectrometer (Applied Biosystems). For MS analyses, 1 000 shots were typically acquired for each spot, whereas for tandem mass spectrometry (MS/MS) analysis, 2 500 shots were acquired. MS/MS analyses were performed using air at a collision energy of 1 kV and a collision gas pressure of 2.061028–3.061027 Torr. The MASCOT search engine (version 1.9, Matrix Science) was used to search all of the tandem mass spectra. GPS Explorer software (version 2.0, Applied Biosystems) was used to create and search files within the MASCOT search engine for peptide and protein identification. Protein identifications were accepted when the observed and predicted pIs (isoelectric points) and Mrs (relative molecular weights) were consistent and scores indicated non-random identifications at a significance level of P < 0.05.
Immunofluorescence staining for verification
We chose spermatozoa protein associated with the nucleus on the X chromosome (SPANXa/d) for verification because of the important role it has in spermatogenesis and spermatozoa maturation. Immunofluorescent staining was performed on the same slide with spermatozoa from both the patient and the healthy control. To permeabilize the cells, the slide was incubated in methanol for 10 min and then rinsed with PBS. To block non-specific protein binding, the slide was incubated in normal goat serum. Slides were incubated with anti-SPANXa/d serum (1:200, Abcam), diluted in PBS with 1% bovine serum albumin and washed with PBS thrice. Then, the slides were incubated with a rhodamine-conjugated secondary antibody (1:100, Santa Cruz Biotechnology) for 1 h at room temperature. The slides were fixed in 4% paraformaldehyde and examined by differential interference contrast.
Results
Sample preparation and DIGE analysis
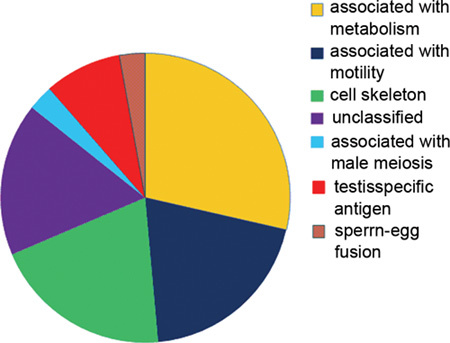
The ultimate goal of this study was to identify a group of proteins that were responsible for globozoospermia. For this purpose, 2-D DIGE was used to generate protein expression profiles from the spermatozoa of a patient with globozoospermia and the spermatozoa of normal controls. Spermatozoa samples were homogenized as described in Section 2.2. In one gel, protein extracts were labelled with either Cy3 (normal) or Cy5 (round-headed) fluorescent dye; in the other gel, the samples were labelled with the opposite dyes. Both gels with a Cy2-labelled sample contained an equal mixture of all samples. The interchangeable use of either Cy3 or Cy5 for either sample has been established in previous studies 20. After 2-D gel electrophoresis, the Cy2, Cy3 and Cy5 channels were individually imaged from the gels using mutually exclusive excitation and emission wavelengths (Figure 1), and the images were analysed using DeCyder as described in section 2.6. In total, 1 056–1 061 unique protein spots were detected by DeCyder Differential Analysis Software. Intergel matching was performed through the inclusion of the internal standard on each gel. Those protein spots that were located at the same position in different gels were matched and a total of 61 protein spots (5.7%) were determined to have a > two fold difference in volume ratio (38 were increased and 23 were decreased) (Figure 2). This indicates that there were remarkably similar protein expression profiles in these two pools. When the volume-ratio threshold was set at threefold, only 35 (3.4%) (Table 1) of the spots showed differential expression between the two pools. The vast majority of proteins fell into three main functional classes: cell skeleton, metabolism and motility (Figure 3).
Figure 1.
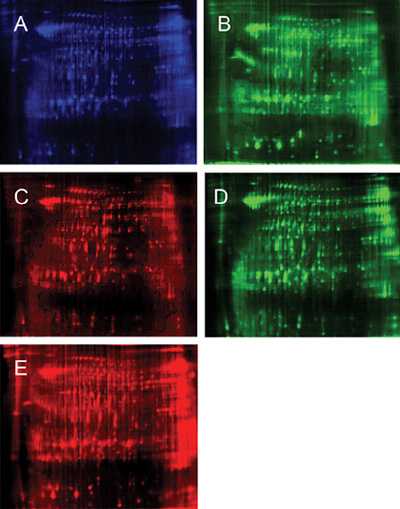
Schematic description of the mixing of separately labelled samples onto the same gel, with subsequent independent Cy-dye imaging for data analysis. The representative two-dimensional (2-D) difference gel electrophoresis (DIGE) image of the two pools (24 cm, PH3-10, normal limits [NL]). Protein extracts (50 mg each) were covalently conjugated to two different fluorescent dyes, and the extracts were pooled and separated on the same gel. The first (horizontal) dimension was a PH 3–10 non-linear focusing gradient and the second vertical dimension was a 12.5% homogenous SDS polyacrylamide gel. Cy2 (blue) was conjugated to proteins used as an internal standard in all samples. In one gel, Cy3 (green) was conjugated to proteins from the round-headed sample (A) and Cy5 (red) was conjugated to proteins from the normal sample (B). In another gel, proteins from the normal sperm sample were conjugated to Cy3 (C) and proteins from the round-headed sperm sample were conjugated to Cy5 (D). (A) Mixed sample labelled with Cy2; (D) Round-headed sperm labelled with Cy3 in gel 1; (c) Normal sperm labelled with Cy5 in gel 1; (D) Normal sperm labelled with Cy3 in gel 2; (E) Round-headed sperm labelled with Cy5 in gel 2.
Figure 2.
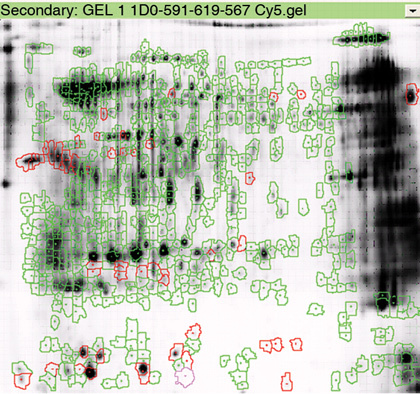
A representative image showing the differential protein expression of normal and round-headed human sperm. The red circles represent the locations of differentially expressed protein spots. Using differential in-gel analysis (DIA), the Cy2, Cy3 and Cy5 images of each gel were merged, spot boundaries were automatically detected and normalized spot volumes (protein abundance) were calculated. Using biological variation analysis (BVA), matching of the protein spots across two gels was performed after several rounds of extensive landmarking and automated matching. After BVA, up to 36 spots were found to have differential expression between the two pools (P < 0.01, AV > 3).
Table 1. Proteins with a threefold difference in expression in round-headed and normal sperm as identified by Matrix-assisted laser desorption/ionization-Time of Flight/Time of Flight*.
| Spot | Protein name | IPI | Mr | Score | Sequence | Change in regulation coverage in round-headed sperm |
|---|---|---|---|---|---|---|
| 1 | A-kinase (PRKA) anchor protein 4 | 157 860 | 95 842 | 234 | 9 | Up |
| 2 | Clusterin precursor | 291 262 | 53 031 | 205 | 9 | Up |
| 3 | ALB protein | 216 773 | 46 442 | 74 | 2 | Up |
| 4 | Testis-specific gene A2 protein | 103 777 | 35 159 | 62 | 4 | Up |
| 5 | Sperm protein | 22 057 | 97 407 | 230 | 9 | Up |
| 6 | Glutathione S-transferase Mu 3 | 246 975 | 26 998 | 154 | 15 | Down |
| 7 | Triosephosphate isomerase 1 variant | 465 028 | 31 057 | 374 | 26 | Down |
| 8 | Isoform 1 of triosephosphate isomerase | 797 687 | 26 938 | 347 | 24 | Down |
| 9 | Isoform 2 of triosephosphate isomerase | 451 401 | 27 451 | 244 | 19 | Down |
| 10 | 30 kDa protein | 746 832 | 29 976 | 237 | 19 | Down |
| 11 | 22 kDa protein | 796 633 | 22 711 | 237 | 17 | Down |
| 12 | RcTPI1 (fragment) | 383 071 | 27 211 | 128 | 13 | Down |
| 13 | Actin, cytoplasmic variant 1 | 21 439 | 42 052 | 157 | 14 | Up |
| 14 | Actin, cytoplasmic variant 2 | 21 440 | 42 108 | 157 | 14 | Up |
| 15 | ACTG1 protein | 794 523 | 28 478 | 114 | 10 | Up |
| 16 | Actin-related protein T2 | 154 776 | 42 074 | 94 | 11 | Down |
| 17 | Outer dense fibre of sperm tails 2 isoform 2 | 642 221 | 73 977 | 75 | 14 | Down |
| 18 | Outer dense fibre of sperm tails 2 isoform 1 | 171 245 | 93 823 | 71 | 15 | Down |
| 19 | Outer dense fibre of sperm tails 2 | 643 280 | 89 161 | 69 | 14 | Down |
| 20 | Outer dense fibre protein 2 | 1 758 | 96 140 | 81 | 18 | Down |
| 21 | Prolactin-inducible protein precursor | 22 974 | 16 847 | 127 | 11 | Up |
| 22 | Isoform 2 of triosephosphate isomerase | 451 401 | 27 451 | 66 | 1 | Down |
| 23 | 94 kDa protein | 22 057 | 96 072 | 38 | 2 | Down |
| 24 | Isoform 1 of A-kinase anchor protein 4 precursor | 157 860 | 95 842 | 119 | 2 | Down |
| 25 | Isoform 2 of A-kinase anchor protein 4 precursor | 333 264 | 94 811 | 119 | 2 | Down |
| 26 | Asparaginase-like 1 protein | 555 734 | 32 406 | 140 | 2 | Down |
| 27 | Isoform 1 of tubulin α-2 chain | 179 709 | 50 612 | 43 | 1 | Down |
| 28 | Isoform 2 of tubulin α-2 chain | 218 345 | 46 935 | 43 | 1 | Down |
| 29 | Similar to α-tubulin | 410 402 | 50 568 | 43 | 1 | Down |
| 30 | α-tubulin isotype H2-α | 552 356 | 50 615 | 43 | 1 | Down |
| 31 | 46 kDa protein | 743 604 | 47 147 | 43 | 1 | Down |
| 32 | Sperm protein associated with the nucleus C on the X chromosome | 6 961 | 11 052 | 47 | 1 | Down |
| 33 | Sperm protein associated with the nucleus on the X chromosome A | 186 783 | 11 088 | 47 | 1 | Down |
| 34 | SPANX-B | 760 825 | 11 834 | 47 | 1 | Down |
| 35 | Sperm acrosome membrane-associated protein 1 precursor | 8 910 | 32 693 | 168 | 3 | Down |
Abbreviations: IPI, international protein index; Mr, relative molecular weight.
The spot number listed in this table is not in accordance with the spot number listed in Figure 3.
Figure 3.
Differentially expressed proteins identified between normal and round-headed spermatozoa. The functional classification is based on Gene Ontology (GO) annotations.
Protein identification
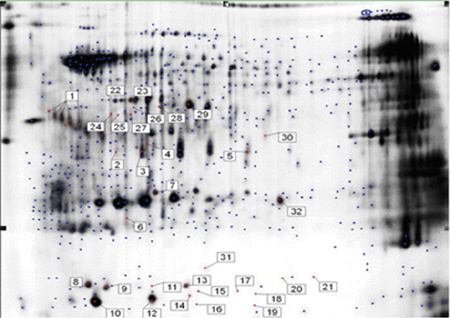
Targeted proteins (Figure 4) were excised from the gel and digested with trypsin as described in Section 2.7. To identify these proteins, MALDI-TOF/TOF MS/MS was used and 35 satisfactory results were obtained. As there might have been the same proteins in two different spots, we identified 36 spots and generated 35 mass spectra results.
Figure 4.
A 2-D gel of human sperm samples showing the location of the differential protein annotations prepared for identification. The gel was stained with coomassie brilliant blue.
Immunofluorescence staining
The characteristics of the SPANXa/d proteins were verified, including their subcellular localizations and their theoretical molecular weights. Immunofluorescence staining was performed to determine the subcellular localization of the SPANXa/d proteins in both normal (A) and patient (B) spermatozoa using anti-SPANX immunoreagents. In normal spermatozoa, SPANXa/d staining was observed in the posterior head, was associated with the cytoplasmic droplet and was localized in the nuclear vacuoles. In globozoospermia, SPANXa/d staining was observed in the posterior head and was associated with the nuclear envelope (Figure 5).
Figure 5.
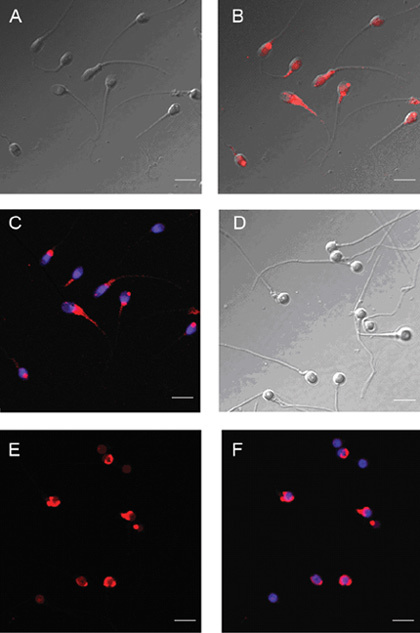
Immunofluorescent/differential interference contrast (DIC) micrographs of normal and patient spermatozoa stained with 4′,6-diamidino-2-phenylindole (DAPI) for DNA (blue) and rhodamine red-X for SPANXa/d protein (red). (A)–(C): Normal spermatozoa; (D)–(F): Globozoospermia; scale bars = 10 μm.
Discussion
In this study, we assessed interclass variations in the spermatozoa proteome by analysing the spermatozoa samples of a patient with globozoospermia and 12 normal spermatozoa samples using 2-D fluorescence DIGE technique. Although there are a number of studies that have used proteomic technologies to analyse the spermatozoa proteome with regard to spermatozoa composition and motility, to our knowledge, this is the first study to assess the qualitative and quantitative differences in protein expression between round-headed and normal spermatozoa. We used DIGE for protein separation and peptide mass fingerprinting by MALDI-TOF/TOF for protein identification. A total of 1061 protein spots were detected using the DeCyder software in which there was differential expression between normal spermatozoa and round-headed spermatozoa, and 61 (5.7%) of these protein spots were up- or downregulated at least twofold in the round-headed spermatozoa as compared with the normal spermatozoa. When the volume-ratio threshold was set at threefold, 36 (3.4%) of the spots showed differential expression between the two pools, with 22 and 14 that were up- and downregulated, respectively. This result is helpful, because it indicates that the absence of acrosome is merely the morphological difference between normal spermatozoa and round-headed spermatozoa, and that, in fact, multiple factors and proteins are implicated in globozoospermatogenesis. The differentially expressed spots were cut out, digested and analysed by the MALDI-TOF method. A total of 35 proteins corresponding to 36 spots were identified using peptide mass profiling and matching the findings to the databases. According to their associated properties, these proteins largely fell into three main functional categories: cell skeleton (20%), metabolism (29%) and motility (21%). One possible explanation is that compared with normal spermatozoa, round-headed spermatozoa are more fragile, and these differentially expressed proteins might be either the cause of round-headed spermatogenesis or the result of round-headed spermatogenesis. Nearly half of the proteins identified in the study are spermatozoa-specific genes. It is well known that spermatozoa-specific proteins have key roles in spermatogenesis, spermatozoa maturation and fertilization. During mammalian fertilization, the acrosomal and plasma membranes of the spermatozoa interact with the egg vestments. Spermatozoa surface molecules located on the plasma membrane are involved in the recognition of the zona pellucida protein during the early process of spermatozoa–egg interaction (primary binding) 22. We identified spermatozoa acrosome membrane-associated protein 1 (SAMP1) in both gels, and it was found to be expressed in lower levels in the globozoospermia patient. SAMP1 is an acrosomal membrane-associated member of the glycosylphosphatidylinisotol-anchored Ly-6/uPAR family of glycoprotein receptors. The Ly-6/uPAR receptors mediate multiple physiological processes, including cellular activation, macrophage migration, trophoblast implantation, angiogenesis and even tumour cell invasion 23. However, the underlying molecular mechanisms responsible for these physiological functions are largely unknown. On the basis of the functions of the other members of this family, such as SAMP14 and SAMP32 24, 25, SAMP1 may serve as orphan receptor on the acrosomal membrane and participate in proteolytic or adhesive events. The fact that this protein was expressed in lower amounts in the globozoospermia patient allowed us to propose the following hypotheses: (1) SAMP1, a basic protein almost exclusively located on the equatorial segment or inner plasma membrane of spermatozoa, appeared to be nearly absent in globozoospermic cells. This finding may indicate that there is an impaired development of the spermatozoa-specific skeleton. SAMP1 may therefore influence both the formation of the acrosome and the shape of the nucleus; (2) SAMP1 may participate in acrosomal vesicle fusion; and (3) as a result of the acrosomeless. At the same time, the SPANX protein and Outer dense fibre protein 2 (ODF2) were found to be expressed in lower levels in globozoospermia. Human SPANX genes comprise of a gene family with five known members (SPANX-A1, A2, B, C and D). These highly similar paralogous genes cluster on the X chromosome at Xq27 and are usually expressed in normal testis and some melanoma cell lines. They are expressed in post-meiotic spermatids and encode highly charged proteins localized to the post-acrosomal perinuclear theca 26, 27. SPANX genes are remarkable in that they have undergone duplication on the X chromosome, while exhibiting, in normal tissues, testis-specific expression and RNA/protein localization exclusively in post-meiotic spermatids 29. The hominoid SPANXa/d proteins are first detected in the nuclear envelope of early round spermatids in the Golgi phase of acrosomal biogenesis and reach their greatest distribution within the nucleus in cap-phase round spermatids before nuclear condensation. As nuclear condensation and elongation proceed, SPANXa/d proteins migrate as distinct post-acrosomal domain of the nuclear envelope towards the base of the nucleus. In mature spermatids, the proteins then associate with the redundant nuclear envelope within the residual cytoplasm. The SPANXa/d domain of the nuclear envelope is thus caudal to the acrosome and reorganizes as acrosome biogenesis progresses, ultimately constricting into the redundant nuclear envelope. In globozoospermia, one or more of the spermatozoa-remodelling mechanisms that occur during spermatogenesis seem to be impaired. Acrosome formation and nuclear elongation have been studied in particular detail in globozoospermia patients. The lower levels of SPANX proteins expressed in globozoospermia patients may explain the absence of the acrosome. Baccetti et al. 9 postulated that the acrosomal granules may be formed in round-headed spermatozoa but subsequently degenerate. He observed that the acrosomic vesicle does attach to the nuclear membrane but degenerates in the late spermatid stage, leaving a pouch of membranes and small vesicles located on top of the nucleus. Moreover, SPANX genes are preserved as functional messages and he suggested that these genes may confer a selective advantage during spermatid nuclear morphogenesis. The lack of SPANX proteins was therefore postulated as a possible cause of this malformation of the acrosome. ODF2, which was also identified in this study, is associated with spermatozoa structure. It is a major protein component of spermatozoa tail outer dense fibres 29, 30. As some evidence suggests that some genetic changes may occur in patients suffering from globozoospermia, lower expression of SPANX proteins in the patient indicates that the expression of the SPANX may be downregulated because of the dysfunction of several unknown genes. The most popular view is that the SPANXa/d protein is responsible for the acrosome biogenesis process. The lower level of expression of SPANXa/d observed in globozoospermia patients, as well as the ectopic expression of SPANX (it is displaced from the base of the nucleus to the nuclear periphery in round-headed spermatozoa, which is where it is located in round spermatids during normal spermatogenesis), indicate that the nuclei of the spermatids stop differentiating in globozoospermia patients, meaning that the acrosome stops forming. There is strong evidence to support the view that SPANXa/d proteins may have an essential role in acrosome formation. In addition, ODF2 was also identified as a widespread component of the centrosomal scaffold and was found to associate preferentially with the appendages of the mother centriole 31.
In 1990, Baltz et al. hypothesized that instead of being directly involved in the induction of progressive motility, the ODF seem to have a modulatory influence on spermatozoa motility. ODF proteins may be necessary for the maintenance of the elastic properties of the spermatozoa tail and may provide tensile strength that is necessary to protect the spermatozoa tail against shearing forces encountered during epididymal transport and especially during ejaculation. Previous studies have also shown that the absence of one or more ODF proteins might be the basis for non-functional tails that could therefore affect spermatozoa motility, leading to the use of the outer fibre protein as a marker of male infertility 33. The lower expression levels of ODF2 that we observed in round-headed spermatozoa could explain the tail fragmentation and low motility of round-headed spermatozoa. Some proteins associated with the cytoskeleton have abnormal expression in round-headed spermatozoa, such as cytoplasmic actin and the tubulin α-2 chain. It is well known that cytoskeletal proteins are involved in many important biological events, including cell movement, signalling, transport and even maintaining membrane shape. The altered expression of these proteins in globozoospermic cells may relate to the pathogenesis of this disorder. In addition, we also identified several enzymes associated with energy metabolism that had lower levels of expression in globozoospermic cells. It is well known that ATP, which is consumed to support spermatozoa movement, is partly produced by glycolysis. Triosephosphate isomerase can catalyse phosphodihydroxyacetone and G3P, and allow them to convert from one structure to the other, which is an important factor in the glycolytic cycle. In our study, triosephosphate isomerase was expressed in lower levels in round-headed spermatozoa. Thus, we presume that the glycolytic cycle may be altered in these spermatozoa, which in turn may lead to a low spermatozoa motility rate in globozoospermic cells. In addition, some hormonal proteins and proteins with unknown functions have been shown to have abnormal expression patterns in globozoospermic cells, including PIP, 94 KDa protein, 40 KDa protein and others. These proteins may have some relationship to the pathogenesis of globozoospermia. However, this hypothesis awaits further confirmation. The present study used a proteomic approach to identify several proteins that are differentially expressed in globozoospermic spermatozoa samples as compared with normal spermatozoa samples. The information obtained may help elucidate the molecular mechanisms underlying spermatogenesis. This information will also be useful in enriching the spermatozoa proteomics database and providing a foundation for further research on single gene function of human round-headed spermatozoa. Owing to the defect in the 2-D DIGE analytic process itself, in this experiment, we inevitably lost many low abundance proteins and some spermatozoa membrane proteins that are hard to extract, and those proteins may also participate in human round-headed spermatozoa formation. The information regarding these proteins needs to be studied further. Human round-headed spermatozoa formation results from multiple factors, rather than merely the absence of one or more proteins. Thus, more research needs to be carried out to reveal its underlying pathophysiology. It is therefore clear that research on globozoospermia is still in its initial stages, and that gene function analysis is needed to reveal the pathological mechanism underlying human round-headed spermatozoa formation.
Acknowledgments
We thank Beijing Proteome Research Center, (Beijing, China) for its enthusiastic technological support and for the theory of 2-D DIGE. We also thank(Changsha, China) College of Life Sciences at Hunan Normal University for supporting the MS technology. Finally, we are very grateful to our collaborators for their help, as well as their valuable discussions and suggestions during the course of this work. This work was supported by two grants from the National Natural Science Foundation of China (NO. 30170480 and NO. 30470884).
References
- Lundin K, Sjögren A, Nilsson L, Hamberger L. Fertilization and pregnancy after intracytoplasmic microinjection of acrosomeless spermatozoa. Fertil Steril. 1994;62:1266–7. [PubMed] [Google Scholar]
- Coetzee K, Windt ML, Menkveld R, Kruger TF, Kitshoff M. An intracytoplasmic sperm injection pregnancy with a globozoospermic male. J Assist Reprod Genet. 2001;18:311–3. doi: 10.1023/A:1016678604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Cha YB, Park JM, Gye MC. Successful pregnancy and delivery from frozen-thawed embryos after intracytoplasmic sperm injection using round-headed spermatozoa and assisted oocyte activation in a globozoospermic patient with mosaic Down syndrome. Fertil Steril. 2001;75:445–7. doi: 10.1016/s0015-0282(00)01698-8. [DOI] [PubMed] [Google Scholar]
- Nardo LG, Sinatra F, Bartoloni G, Zafarana S, Nardo F. Ultrastructural features and ICSI treatment of severe teratozoospermia: report of two human cases of globozoospermia. Eur J Obstet Gynecol Reprod Biol. 2002;104:40–2. doi: 10.1016/s0301-2115(01)00602-9. [DOI] [PubMed] [Google Scholar]
- Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20:2237–41. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- Stone S, O'Mahony F, Khalaf Y, Taylor A, Braude P. A normal livebirth after intracytoplasmic sperm injection for globozoospermia without assisted oocyte activation: case report. Hum Reprod. 2000;15:139–41. doi: 10.1093/humrep/15.1.139. [DOI] [PubMed] [Google Scholar]
- Kilani Z, Ismail R, Ghunaim S, Mohamed H, Hughes D, et al. Evaluation and treatment of familial globozoospermia in five brothers. Fertil Steril. 2004;82:1436–9. doi: 10.1016/j.fertnstert.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Holstein AF, Schirren C, Schirren CG. Human spermatids and spermatozoa lacking acrosomes. J Reprod Fertil. 1973;35:489–91. doi: 10.1530/jrf.0.0350489. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Collodel G, Piomboni P. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9) J Submicrosc Cytol Pathol. 1996;28:587–96. [PubMed] [Google Scholar]
- Vicari E, Perdichizzi A, De Palma A, Burrello N, D'Agata R, et al. Globozoospermia is associated with chromatin structure abnormalities: case report. Hum Reprod. 2002;17:2128–33. doi: 10.1093/humrep/17.8.2128. [DOI] [PubMed] [Google Scholar]
- Machev N, Gosset P, Viville S. Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: teratozoospermia. Cytogenet Genome Res. 2005;111:352–7. doi: 10.1159/000086910. [DOI] [PubMed] [Google Scholar]
- Rubes J, Lowe X, Moore D, Perreault S, Slott V, et al. Smoking cigarettes is associated with increased sperm disomy in teenage men. Fertil Steril. 1998;70:715–23. doi: 10.1016/s0015-0282(98)00261-1. [DOI] [PubMed] [Google Scholar]
- Kilani Z, Ismail R, Ghunaim S, Mohamed H, Hughes D, et al. Evaluation and treatment of familial globozoospermia in five brothers. Fertil Steril. 2004;82:1436–9. doi: 10.1016/j.fertnstert.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha' catalytic subunit. Nat Genet. 1999;23:118–21. doi: 10.1038/12729. [DOI] [PubMed] [Google Scholar]
- Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, van Deursen JM. Lack of acrosome formation in Hrb-deficient mice. Science. 2001;294:1531–3. doi: 10.1126/science.1063665. [DOI] [PubMed] [Google Scholar]
- Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, et al. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci USA. 2002;99:11211–6. doi: 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Ke-Li, Fan Li-Qing, Zhu Wen-Bing, LU Guang-Xiu. Isolation and identification of differentially 2 expressed proteins in round-headed human spermatozoa. Prog Biochem Biophys. 2003. p. 30.
- Naaby-Hansen S, Flickinger CJ, Herr JC. Two-dimensional gel electrophoretic analysis of vectorially labeled surface proteins of human spermatozoa. Biol Reprod. 1997;56:771–87. doi: 10.1095/biolreprod56.3.771. [DOI] [PubMed] [Google Scholar]
- Liang CR, Leow CK, Neo JC, Tan GS, Lo SL, et al. Proteome analysis of human hepatocellular carcinoma tissues by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics. 2005;5:2258–71. doi: 10.1002/pmic.200401256. [DOI] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–33. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- Kolkman A, Dirksen EH, Slijper M, Heck AJ. Double standards in quantitative proteomics: direct comparative assessment of difference in gel electrophoresis and metabolic stable isotope labeling. Mol Cell Proteomics. 2005;4:255–66. doi: 10.1074/mcp.M400121-MCP200. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Litscher ES. Sperm–egg recognition mechanisms in mammals. Curr Top Dev Biol. 1995;30:1–19. doi: 10.1016/s0070-2153(08)60562-1. [DOI] [PubMed] [Google Scholar]
- Gumley TP, McKenzie IF, Sandrin MS. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol Cell Biol. 1995;73:277–96. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- Shetty J, Wolkowicz MJ, Digilio LC, Klotz KL, Jayes FL, et al. SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm–egg interaction. J Biol Chem. 2003;278:30506–15. doi: 10.1074/jbc.M301713200. [DOI] [PubMed] [Google Scholar]
- Hao Z, Wolkowicz MJ, Shetty J, Klotz K, Bolling L, et al. SAMP32, a testis-specific, isoantigenic sperm acrosomal membrane-associated protein. Biol Reprod. 2002;66:735–44. doi: 10.1095/biolreprod66.3.735. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Iguchi N, Kaneko Y, Tanaka H, Nishimune Y. Characterization of a novel postacrosomal perinuclear theca-specific protein, CYPT1. Biol Reprod. 2004;71:1927–35. doi: 10.1095/biolreprod.104.032789. [DOI] [PubMed] [Google Scholar]
- Hansen MA, Nielsen JE, Tanaka M, Almstrup K, Skakkebaek NE, et al. Identification and expression profiling of 10 novel spermatid expressed CYPT genes. Mol Reprod Dev. 2006;73:568–79. doi: 10.1002/mrd.20463. [DOI] [PubMed] [Google Scholar]
- Zendman AJ, Zschocke J, van Kraats AA, de Wit NJ, Kurpisz M, et al. The human SPANX multigene family: genomic organization, alignment and expression in male germ cells and tumor cell lines. Gene. 2003;309:125–33. doi: 10.1016/s0378-1119(03)00497-9. [DOI] [PubMed] [Google Scholar]
- Brohmann H, Pinnecke S, Hoyer-Fender S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J Biol Chem. 1997;272:10327–32. doi: 10.1074/jbc.272.15.10327. [DOI] [PubMed] [Google Scholar]
- Schalles U, Shao X, van der Hoorn FA, Oko R. Developmental expression of the 84-kDa ODF sperm protein: localization to both the cortex and medulla of outer dense fibers and to the connecting piece. Dev Biol. 1998;199:250–60. doi: 10.1006/dbio.1998.8931. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Yamane Y, Okanoue T, Tsukita S, Tsukita S. Outer dense fiber 2 is a widespread centrosome scaffold component preferentially associated with mother centrioles: its identification from isolated centrosomes. Mol Biol Cell. 2001;12:1687–97. doi: 10.1091/mbc.12.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz JM, Williams PO, Cone RA. Dense fibers protect mammalian sperm against damage. Biol Reprod. 1990;43:485–91. doi: 10.1095/biolreprod43.3.485. [DOI] [PubMed] [Google Scholar]
- Petersen C, Füzesi L, Hoyer-Fender S. Outer dense fibre proteins from human sperm tail: molecular cloning and expression analyses of two cDNA transcripts encoding proteins of approximately 70 kDa. Mol Hum Reprod. 1999;5:627–35. doi: 10.1093/molehr/5.7.627. [DOI] [PubMed] [Google Scholar]


