Fig. 1.
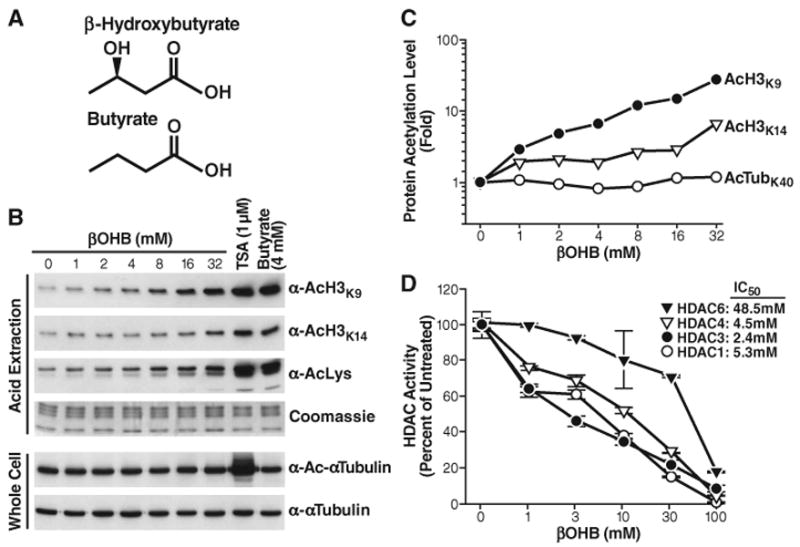
Inhibition of HDACs by βOHB in vitro and in vivo. (A) Structures of β-hydroxybutyrate and butyrate. (B) Effect of βOHB, TSA, or butyrate on acetylation of histone H3 and tubulin. HEK293 cells were treated with the indicated concentrations of drugs for 8 hours. Histones were acid-extracted, and their acetylation was assessed by protein immunoblotting with anti-AcH3K9, anti-AcH3K14, or anti-acetyllysine (AcLys). Proteins from whole-cell extracts were analyzed by immunoblotting with antibodies to α-tubulin or Ac-α-Tubulin. (C) Quantification of acetylation levels from blots in (B), shown relative to untreated cells (βOHB 0 mM). (D) Inhibition of immunopurified HDACs by βOHB in vitro. Flag-tagged HDACs were expressed in HEK293 cells, immunoprecipitated, and incubated in vitro with a 3H-labeled acetylated histone H4 peptide and the indicated concentrations of βOHB. HDAC activity is relative to the activity of each enzyme without βOHB. The IC50 values of βOHB are shown.
