Figure 7.
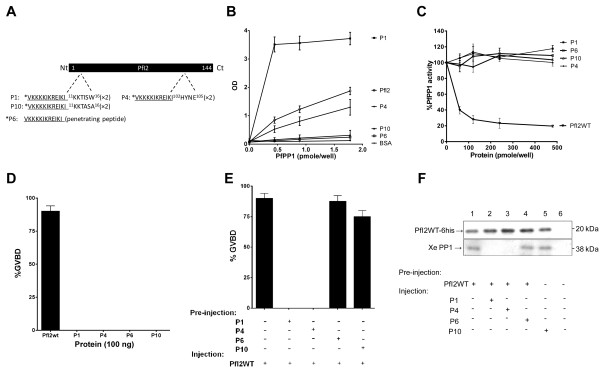
Role of synthetic peptides derived from PfI2. A. Schematic representation of PfI2 sequence and P1 and P4 peptides derived, respectively, from the RVxF (11KKTISW16) and HYNE (102HYNE105) domains. P6 (penetrating peptide) and P10 (P1 mutated version) were used as negative controls. B. Interaction studies of P1, P4, P6, P10 with His6-PfPP1 in vitro using ELISA based quantification of binding capacity. Increasing quantities of biotinylated His6-PfPP1 were added to wells coated with peptides (5 μg/well) or recombinant PfI2WT protein (5 μg/well) used as positive control. Results are representative of 4 independent experiments. C. Effect of P1, P4, P6 and P10 on PfPP1 phosphatase activity. Recombinant His6-PfPP1 at 27.03 nM (2.5 μg) was pre-incubated for 30 min at 37°C with different concentrations of peptides or PfI2WT (positive control) before the addition of pNPP. Results presented as % of relative increase or decrease are means ± SEM of four independent experiments performed in duplicate. D. Initiation of G2/M in Xenopus oocytes by peptides. Appearance of GVBD was monitored for 15 h after injection, and values are presented as percentages. Each experiment was performed using a set of 20 oocytes and was repeated at least three times. PfI2WT injection was used as positive control. E. Percentage of GVBD induced by the pre-injection of P1, P4, P6 or P10 (100 ng) 1 hour before PfI2WT injection (100 ng). PfI2WT injection was used as a positive control. F. Abolition of PfI2WT/XePP1 interaction after P1 and P4 peptides pre-injection. Extracts from oocytes pre-injected or not with peptides followed by PfI2WT injection were immunoprecipitated with anti-His mAb. Immunoblot analysis revealed with specific anti-XePP1 antibodies revealed the presence of XePP1 in the complex in P6 and P10 pre-injected extracts (lanes 4 and 5) and in non-pre-injected extracts (lane 1) but not in the P1 and P4 pre-injected extracts (lanes 2 and 3).
