Abstract
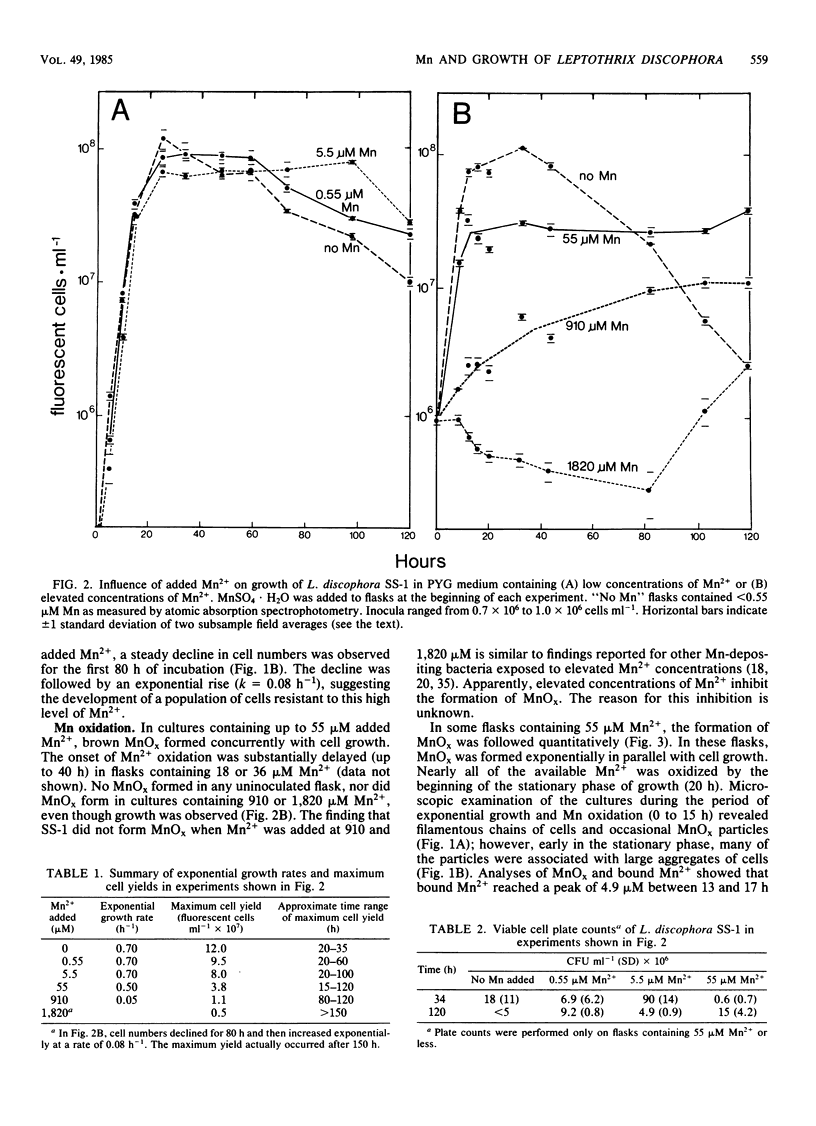
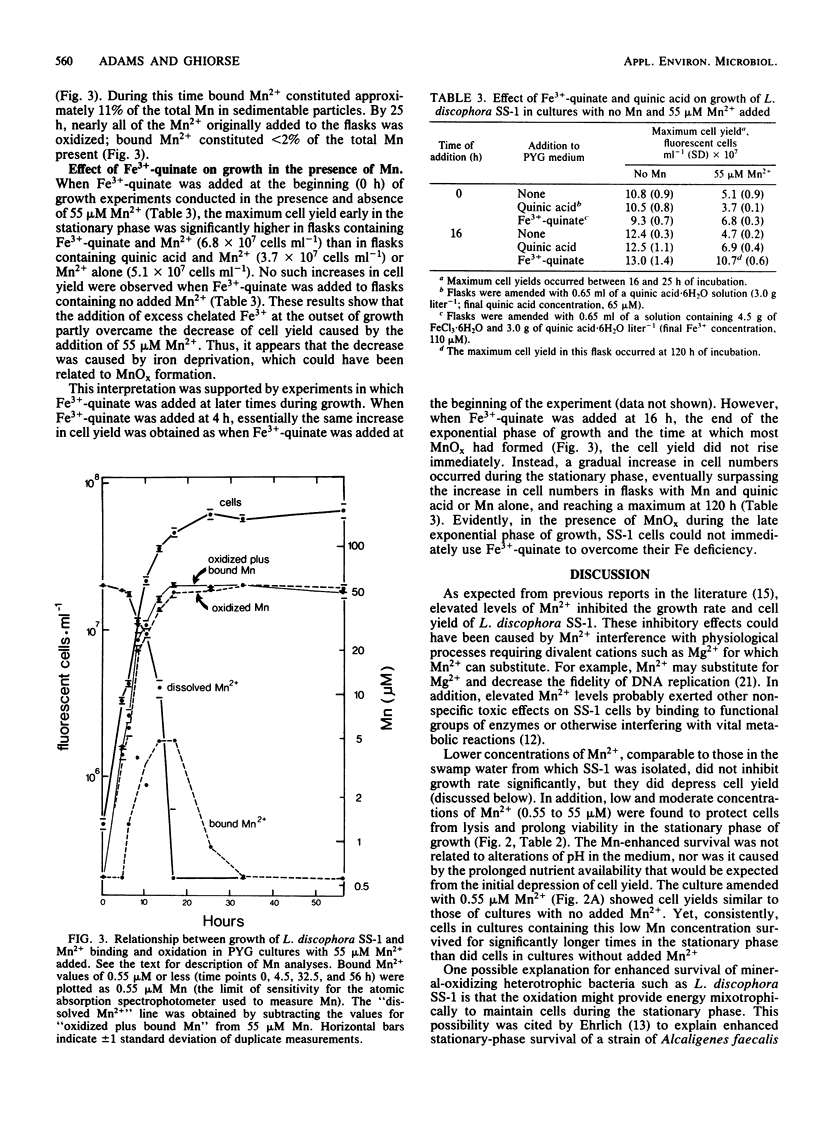
Mn2+ exerted various effects on the growth of Leptothrix discophora strain SS-1 in batch cultures depending on the concentration added to the medium. Concentrations of 0.55 to 5.5 μM Mn2+, comparable to those in the environment from which strain SS-1 was isolated, decreased cell yield and prolonged stationary-phase survival, but did not affect growth rate. Elevated concentrations of 55 to 910 μM Mn2+ also decreased cell yield and prolonged survival, but growth rate was decreased as well. The addition of 1,820 μM Mn2+ caused a decline in cell numbers followed by an exponential rise after 80 h of incubation, indicating the development of a population of cells resistant to Mn2+ toxicity. When 360 μM Mn2+ or less was added to growth flasks, Mn2+ was oxidized to manganese oxide (MnOx, where x is ∼2), which appeared as brown particles in the medium. Quantification of Mn oxidation during growth of cultures to which 55 μM Mn2+ was added showed that nearly all of the Mn2+ was oxidized by the beginning of the stationary phase of growth (15 to 25 h). This result suggested that the decrease in cell yield observed at low and moderate concentrations of Mn2+ was related to the formation of MnOx, which may have bound cationic nutrients essential to the growth of SS-1. The addition of excess Fe3+ to cultures containing 55 μM Mn2+ increased cell yield to levels near those found in cultures with no added Mn2+, indicating that iron deprivation by MnOx was at least partly responsible for the decreased cell yield.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. H., Stokes J. L. Stimulation of heterotrophic and autotrophic growth of Sphaerotilus discophorus by manganous ions. Antonie Van Leeuwenhoek. 1971;37(4):519–528. doi: 10.1007/BF02218522. [DOI] [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984 Apr;158(1):1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys. 1982 May;215(2):589–596. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981 Jan;145(1):442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981 Jun;146(3):928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P., Maratea D., Wolfe R. S. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979 Nov;140(2):720–729. doi: 10.1128/jb.140.2.720-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C. Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol. 1984;38:515–550. doi: 10.1146/annurev.mi.38.100184.002503. [DOI] [PubMed] [Google Scholar]
- Ghiorse W. C., Hirsch P. Isolation and properties of ferromanganese-depositing budding bacteria from baltic sea ferromanganese concretions. Appl Environ Microbiol. 1982 Jun;43(6):1464–1472. doi: 10.1128/aem.43.6.1464-1472.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. F., Keener S., Guidotti S., Branscomb E. W. On the enzymatic basis for mutagenesis by manganese. J Biol Chem. 1983 Mar 25;258(6):3469–3475. [PubMed] [Google Scholar]
- Hajj H., Makemson J. Determination of growth of Sphaerotilus discophorus in the presence of manganese. Appl Environ Microbiol. 1976 Nov;32(5):699–702. doi: 10.1128/aem.32.5.699-702.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Sigda J., Kapuscinski R., Mitchell R. Statistical analysis of the direct count method for enumerating bacteria. Appl Environ Microbiol. 1982 Aug;44(2):376–382. doi: 10.1128/aem.44.2.376-382.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Functional significance of manganese catalase in Lactobacillus plantarum. J Bacteriol. 1983 Aug;155(2):742–746. doi: 10.1128/jb.155.2.742-746.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983 May 25;258(10):6015–6019. [PubMed] [Google Scholar]
- Lance E. A., Rhodes C. W., 3rd, Nakon R. Free metal ion depletion by "Good's" buffers. III. N-(2-acetamido)iminodiacetic acid, 2:1 complexes with zinc(II), cobalt(II), nickel(II), and copper(II); amide deprotonation by Zn(II), Co(II), and Cu(II). Anal Biochem. 1983 Sep;133(2):492–501. doi: 10.1016/0003-2697(83)90115-x. [DOI] [PubMed] [Google Scholar]
- Rogers S. R., Anderson J. J. Role of iron deposition in Sphaerotilus discophorus. J Bacteriol. 1976 Apr;126(1):264–271. doi: 10.1128/jb.126.1.264-271.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson R. A., Nealson K. H. Manganese binding and oxidation by spores of a marine bacillus. J Bacteriol. 1982 Aug;151(2):1027–1034. doi: 10.1128/jb.151.2.1027-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson R. A., Tebo B. M., Nealson K. H. Use of poisons in determination of microbial manganese binding rates in seawater. Appl Environ Microbiol. 1984 Apr;47(4):740–745. doi: 10.1128/aem.47.4.740-745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- van Veen W. L., Mulder E. G., Deinema M. H. The Sphaerotilus-Leptothrix group of bacteria. Microbiol Rev. 1978 Jun;42(2):329–356. doi: 10.1128/mr.42.2.329-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]