Figure 3. The knockdown efficacy of a range of concentrations of siRab41(4).
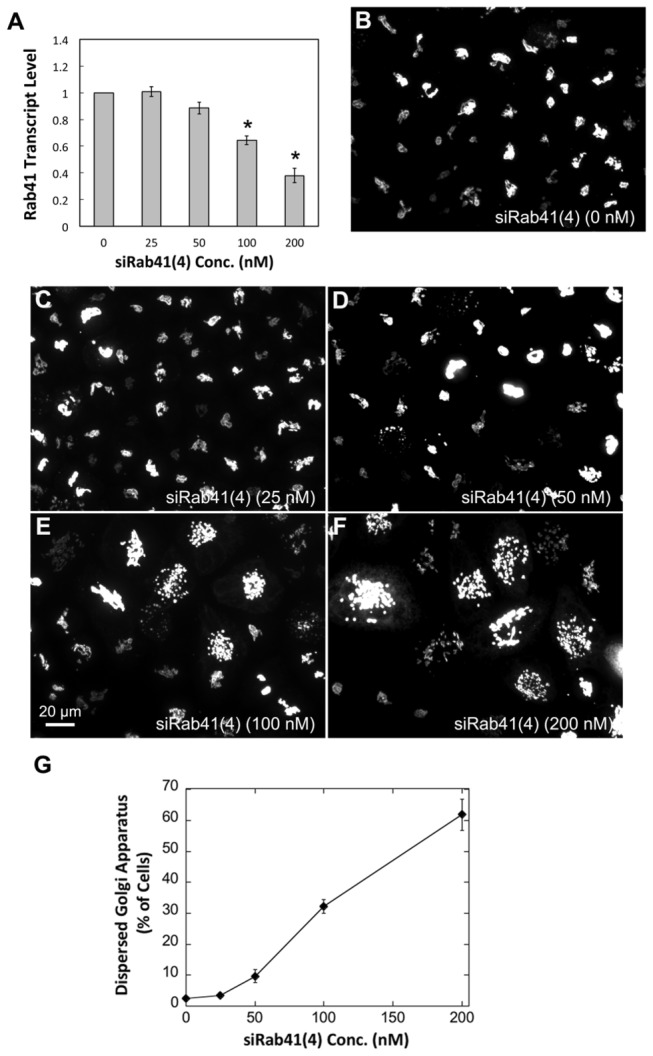
HeLa cells stably expressing GalNAcT2-GFP were transfected with siRab41(4) at concentration of 0, 25, 50, 100 or 200 nM. HeLa cells were fixed or collected 96 h post initial transfection. Total RNA was isolated and transcribed to cDNA. Real-time PCR showed that, in cells treated by 100 nM and 200 nM siRab41(4), Rab41 transcript level was decreased ~30 and 60% relative to GADPH (as control), respectively (A, asterisks). Error bars in (A) represent the mean ± SEM of three replicates. Most of the cells transfected with 0, 25 or 50 nM siRab41(4) displayed normal Golgi ribbon structure (B–D). However, if the concentration of siRab41(4) was increased to 100 nM or 200 nM, fragmented Golgi apparatus was observed in many more cells (E and F). (G) Quantitatively, >30% cells treated with 100 nM siRab41(4) had clustered punctate Golgi distribution, and >60% cells transfected with 200 nM siRab41(4) displayed disrupted Golgi apparatus. Error bars represent the mean ± SEM of three replicates. ~100 cells were assayed for each condition and individual replicate.
