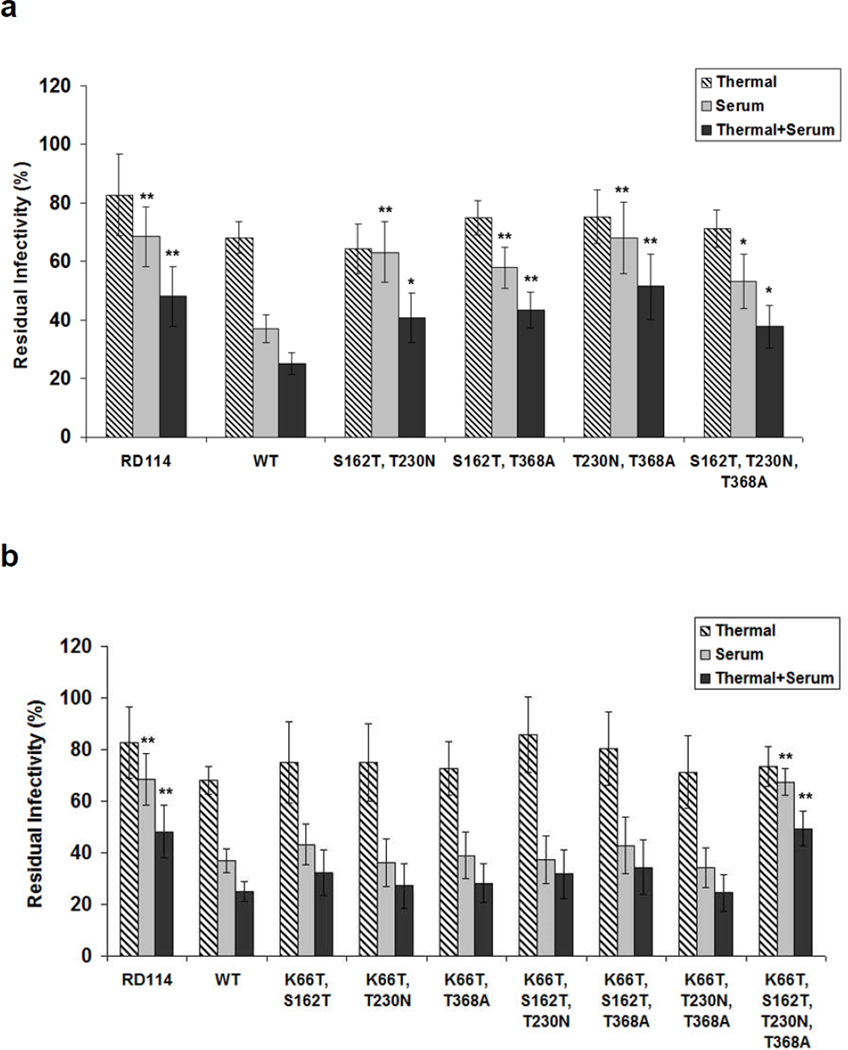
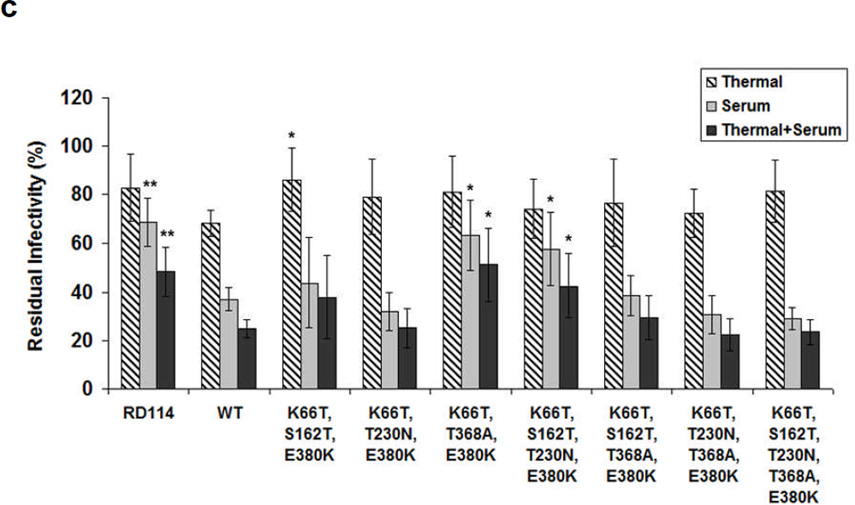
Figure 4. Human serum neutralization and thermostability of retroviral vectors pseudotyped with VSV-G variants that combine several ‘hot spot mutations’.
VSV-G mutants with combined beneficial mutations were generated by site-directed mutagenesis. The amounts of viral vectors were normalized based on VSV-G ELISA assay. Thermal effects, human serum neutralization, and combined serum neutralization and thermal effects were determined by quantifying relative titers after incubation with PBS at 37°C for 1 hr compared to those without incubation at 37°C, after incubation with human serum at 37°C for 1 hr compared to those after incubation with PBS at 37°C for 1 hr, and after incubation with human serum at 37°C for 1 hr compared to those without incubation at 37°C, respectively. (a) Variants contained ‘hot spot mutations’ for human serum resistance. (b) K66T and (c) E380K were added to enhance the thermal stability of retroviral vectors. Error bars denote SD (n = 4). * and ** indicate statistical differences of P < 0.05 and P < 0.01, respectively, compared to the wild type VSV-G, as determined using an one-way ANOVA.