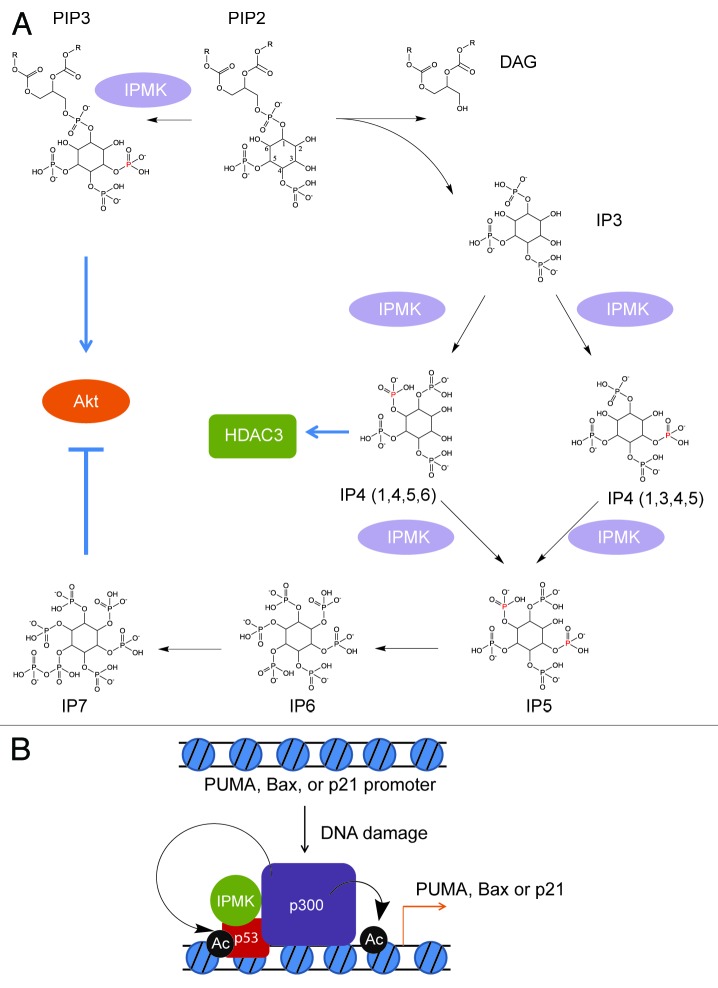
Figure 1. Inositol polyphosphate multikinase (IPMK) is a multifunctional enzyme with PI3-kinase, IP3-kinase and catalytically independent activities. (A) IPMK is a PI3-kinase which phosphorylates PIP2 into PIP3, serving as a physiologic activator of Akt/PKB. It also possesses IP3-kinase activity, converting IP3 into IP4 (1,3,4,5) or IP4 (1,4,5,6). Whether the phosphorylation of IP3 at the 3-position or the 6-position is physiologically regulated remains to be fully elucidated. One of the IP4 isoforms, IP4 (1,4,5,6), has been co-crystallized at the interface of HDAC3 and the DAD domain of NCor/SMRT, enhancing HDAC3 activity in vitro.3 IPMK subsequently phosphorylates IP4 into IP5, and is the rate-limiting enzyme for this metabolite. Thus, IPMK serves as the gate-keeping enzyme for the synthesis of all higher inositol polyphosphate species, including inositol pyrophosphates, which have been implicated in diverse physiologic processes. (B) Independent of catalytic activity, IPMK binds to p53 and enhances its association with p300. The enhancement of p300s histone acetyltransferase activity by IPMK leads to increased acetylation of p53 and histone H3, as well as p53 association to target promoters. This augmentation of p53-dependent gene transcription enhances cell death.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.