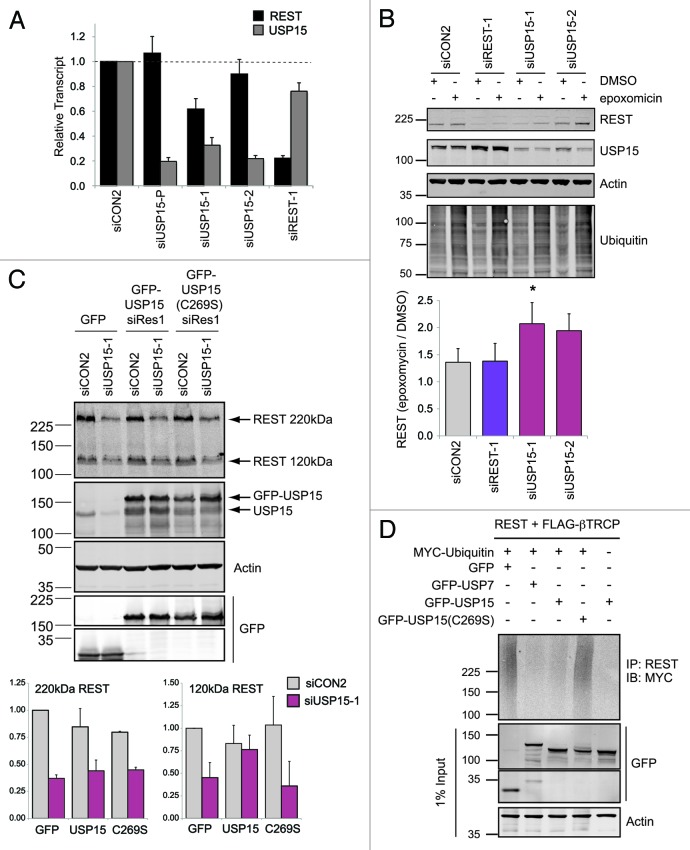
Figure 3. USP15 can exert a post-translational effect on REST. (A) The effect of USP15 depletion on REST protein abundance is not predicated on reduction of REST mRNA. A549 cells were transfected with siRNAs as indicated and RNA was prepared 72 h later. REST or USP15 transcripts were quantified relative to β-actin (ACTB) by qRT-PCR; the mean of four independent experiments is shown (bars show standard error). (B) The loss of REST in USP15-depleted cells can be rescued by proteasome inhibition. A549 cells were transfected with siRNA as indicated for 72 h and treated with 50 nM epoxomicin for the final 6 h prior to whole-cell lysis and immunoblotting. Representative blots are shown with the mean fold induction of REST following proteasome inhibition plotted below [siUSP15-1, n = 5, *p = 0.045 (epoxomicin/DMSO); siUSP15-2, n = 3, p = 0.098; bars show standard error]. (C) Catalytically active USP15 can partially rescue levels of nascent REST. HEK-293T cells were transfected with the indicated plasmids 24 h after treatment with USP15 siRNA or control reagents. Whole-cell protein extracts were prepared 48 h later for immunoblotting. The amount of unglycosylated 120 kDa REST or O-glycosylated 220 kDa REST were determined from three independent experiments and normalized to actin; all values are expressed relative to cells transfected with control siRNA and a plasmid expressing GFP alone (lane 1) (n = 3, error bars show standard deviation). (D) Catalytic activity is required for USP15 to reverse ubiquitylation of REST. HEK-293T cells were transfected with the indicated constructs for 48 h and treated with epoxomicin for the last 6 h, before immunoprecipitation with an anti-REST antibody and immunoblotting for myc-tagged ubiquitin. A representative blot from two independent experiments is shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.