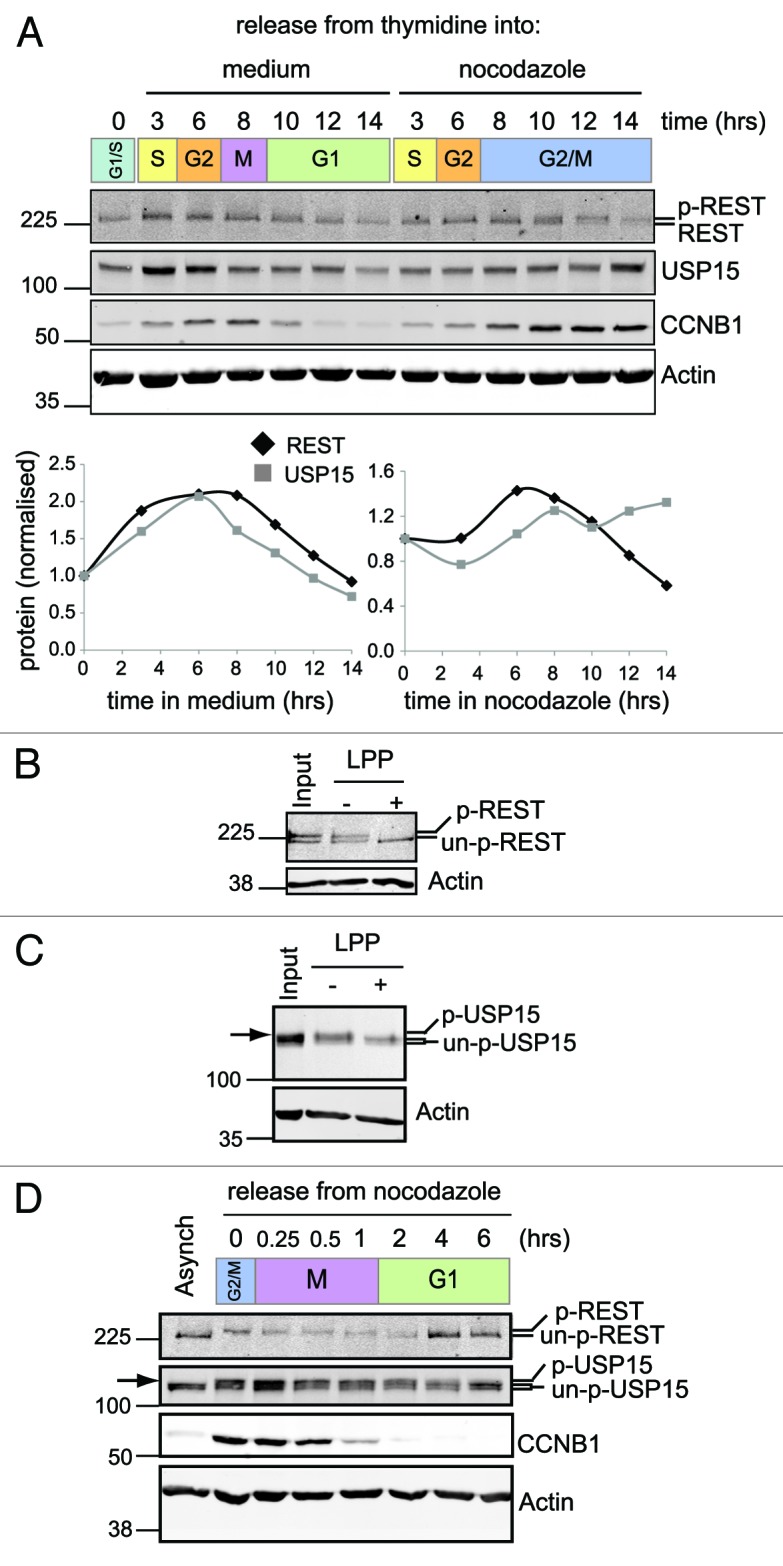
Figure 6. REST is degraded at mitosis and resynthesised as cells enter G1. (A) REST accumulates on release from G1/S arrest and degrades in cells arrested at G2/M. A549 cells were synchronized with thymidine and released from G1/S into media (left) or media containing nocodazole (right). Protein expression was monitored by immunoblotting over 14 h. Total REST and USP15 were quantified from duplicate gels, normalized to actin and plotted relative to the level before release from the thymidine block. (B and C) Phosphorylated forms of both REST (B) and USP15 (C) accumulate during prometaphase arrest. Thymidine synchronized A549 cells were released into nocodazole for 12 h and lysed with E1A buffer. Protein extract was incubated in buffer alone (−) or with lambda protein phosphatase (LPP, +) prior to immunoblotting for REST or USP15; untreated extract (input) is shown for comparison. P-USP15 is indicated with an arrow, above the USP15 doublet seen in asynchronous cells. (D) Phosphorylated REST degrades at mitosis and unphosphorylated REST accumulates as cells enter G1. A549 cells were synchronized with thymidine and then arrested in nocodazole for 14 h before shaking off the mitotic cells and releasing them from G2/M arrest (0 h) into fresh medium. Protein expression was monitored by immunoblotting.
