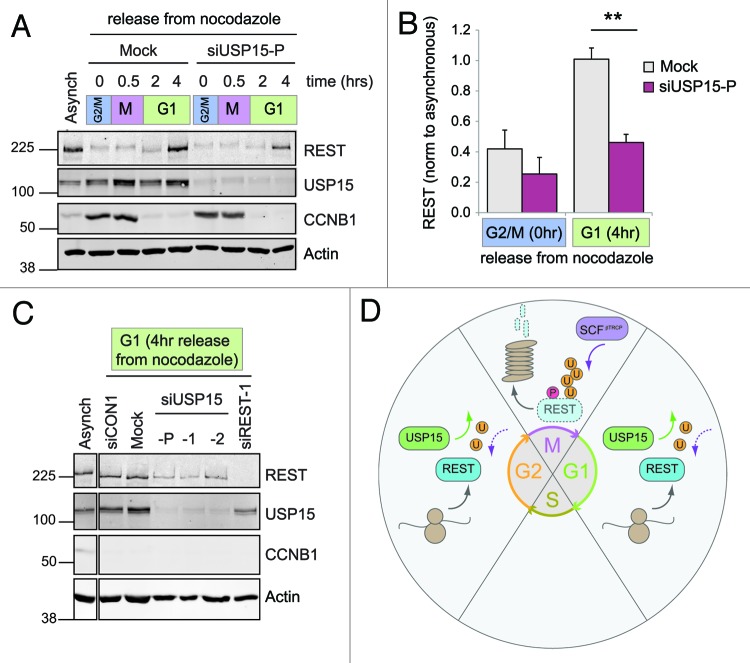
Figure 7. USP15 is required for the recovery of REST levels on mitotic exit. (A) USP15 depletion does not prevent degradation of phosphorylated REST at mitosis but limits its recovery. A549 were transfected with siRNA prior to thymidine synchronization and then arrested in nocodazole for 14 h before shaking off the mitotic cells and releasing from G2/M arrest (0 h). Protein expression was monitored for 4 h, and a representative immunoblot is shown. (B) USP15 depletion impairs recovery of REST at mitotic exit. Cells were treated as described in (A) and extracts collected for immunoblotting before release from G2/M (0 h) or in early G1 (4 h). Mean quantification of REST normalized to actin is plotted, relative to that in asynchronous cells, for four independent experiments (bars show standard error, **p = 0.0005). (C) Individual USP15 siRNAs recapitulate the block on REST accumulation in early G1. Cells were transfected with siRNAs as indicated, then treated and extracts collected as in (B). A representative immunoblot from two independent experiments is shown at 4 h post-release from nocodazole. (D) A model for REST regulation by USP15. USP15 does not oppose the phosphorylation-dependent eradication of REST at mitosis (M). Instead, during G1 and G2, USP15 counteracts ubiquitylation of newly synthesized REST, allowing its levels to accumulate.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.