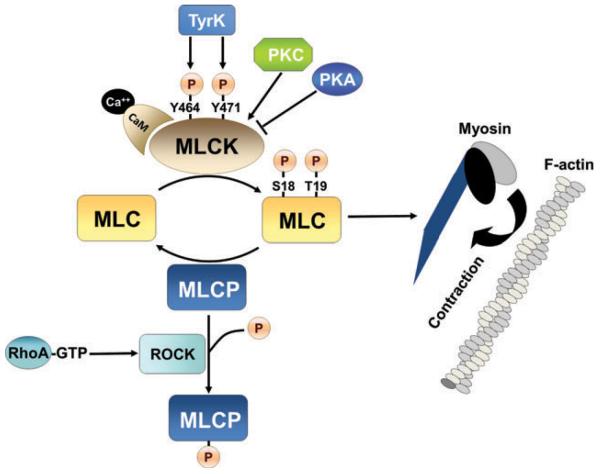
Figure 1.
Myosin light chain kinase (MLCK)-dependent control of actin-myosin contraction in endothelium. Increased myosin light chain (MLC) phosphorylation at Ser-18 and Tyr-19 in response to MLCK activation or myosin light chain phosphatase (MLCP) inhibition increases MLC ATPase-driven force generation relative to actin. MLCK activity is increased by Ca2+-calmodulin binding, and/or by kinase-dependent phosphorylation including phosphorylation by protein kinase C (PKC) or tyrosine kinase phosphorylation at Tyr-464 and Tyr-471. MLCK activity is decreased in response to protein kinase A (PKA) activity. Inhibition of MLCP is achieved by ROCK activation downstream of RhoA activation, resulting in phosphorylation-dependent inhibition of MLCP.