Figure 1.
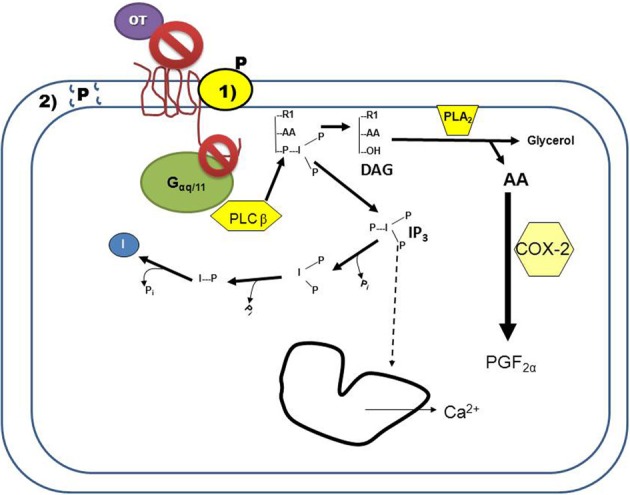
Progesterone (P) inhibition of oxytocin receptor (OXTR) signaling in endometrial cells. Binding of oxytocin (OT) to the OXTR activates the G-protein, Gαq/11. The activated G-protein phosphorylates the enzyme phospholipase Cβ (PLCβ), which cleaves phosphatidylinositol 4,5-bisphosphate into DAG and inositol 1,4,5-trisphosphate (IP3). Released IP3 induces intracellular calcium release (Ca2+) from the endoplasmic reticulum, and is then recycled into free inositol. Free arachadonic acid (AA) is cleaved from DAG by phospholipase A2 (PLA2) and then converted by cyclooxygenase 2 (COX2) into prostaglandin F2α (PGF2α). Blockage of this signaling cascade by P can occur by two mechanisms: (1) P binding to a membrane-associated binding protein that interacts with the OXTR, resulting in conformational changes to the receptor such that OT is not able to bind and/or the OXTR is unable to interact with the G-protein. (2) P overloading of the plasma membrane results in changes to membrane fluidity, preventing the OXTR from interacting with the G-protein. Both of these mechanisms of action would result in P-mediated decreased signaling of the OXTR.
