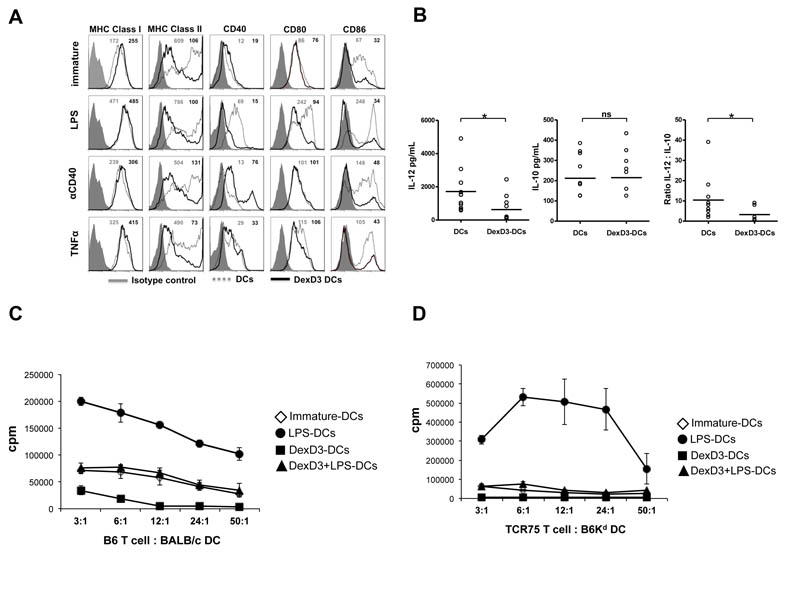
Figure 1. DexD3-DCs are refractory to maturation and are inefficient APCs.
BALB/c-DCs were grown in the presence or absence of Dex and D3 (DexD3-DCs) for 7 days. LPS, TNFα or anti-CD40 antibody were added for the last 24 hours of culture. Control drug treated DCs received no maturation stimuli.
(A) DCs were stained for the expression of MHC class I, class II, CD40, CD80 and CD86 molecules using specific antibodies and analysed by flow cytometry. Isotype controls are shown as grey histograms. Untreated DexD3 treated DCs are shown as grey dashed lines whilst DexD3-DCs are shown as solid black lines. The MFI of both untreated (grey) and treated (black) is shown in the top right hand side of each panel. One representative experiment of 13 (LPS) and 3 (TNFα and anti-CD40 antibody) is shown.
(B) Immature and DexD3-DCs were co-cultured for 18 hours, alone or with a CD40L-expressing cell line, supernatants from these co-cultures were used to detect production of IL-10 and IL-12p40/p70 by ELISA (*p<0.05).
(C) DCs derived from BALB/c, treated or not with DexD3 and LPS [(LPS-matured DCs (closed circles), immature DCs (open diamonds), DexD3+LPS-DCs (closed triangles) and DexD3-DCs (closed squares)] were co-cultured with 1×104 CD4+ T cells isolated from B6 mice at different ratios. T cell proliferation was measured after 72 hours following addition of 3H thymidine for the last 16 hours of culture. Proliferation is expressed as counts per minute (cpm) ± SD. One representative experiment is shown out of 3 performed.
(D) DCs derived from B6Kd mice, treated or not with DexD3 and LPS [(LPS-matured DCs (closed circles), immature DCs (open diamonds), DexD3+LPS-DCs (closed triangles) and DexD3-DCs (closed squares)] were co-cultured with 1×104 CD4+ T cells isolated from TCR75 Rag−/− mice at different ratios. T cell proliferation was measured after 72 hours following addition of 3H thymidine for the last 16 hours of culture. Proliferation is expressed as counts per minute (cpm) ± SD. One representative experiment is shown out of 3 performed.