Abstract
Background
Here, we aimed to investigate the pharmacokinetics of abacavir and carbovir triphosphate (CBV-TP) with darunavir/ritonavir 900/100 mg once daily or raltegravir 400 mg twice daily.
Methods
HIV-infected subjects on abacavir (600 mg once daily) underwent steady-state pharmacokinetic assessments without and with darunavir/ritonavir or raltegravir. Within-subject changes in plasma and intracellular pharmacokinetic parameters were evaluated by geometric mean ratios (GMRs) and 90% CIs.
Results
A total of 19 patients completed the study. With darunavir/ritonavir (versus abacavir alone), abacavir GMRs (90% CI) were 0.73 (0.66, 0.80), 0.62 (0.50, 0.77) and 0.78 (0.69, 0.87) for area under the curve (AUC), trough concentration (Ctrough), and maximum concentration (Cmax), respectively. With raltegravir, they were 1.03 (0.97, 1.10), 0.83 (0.62, 1.11) and 1.06 (0.95, 1.181, respectively. Intracellular CBV-TP GMRs (90% CI) were 0.88 (0.72, 1.07), 0.68 (0.48, 0.95) and 0.98 (0.79, 1.23) for AUC, Ctrough and Cmax, respectively, with darunavir/ritonavir, and 0.96 (0.76, 1.20),0.57 (0.33, 1.00) and 1.07 (0.85, 1.35), respectively, with raltegravir.
Conclusions
There was a 27% decrease in abacavir plasma exposure with darunavir/ritonavir and no changes with raltegravir. CBV-TP Ctrough was significantly decreased with darunavir/ritonavir (32%) and showed a high inter-individual variability with raltegravir.
Introduction
Guidelines for the treatment of HN infection recommend that initial therapy should include two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) plus one non-nucleoside reverse transcriptase inhibitor (NNRTI) or a ritonavir-boosted protease inhibitor [1]. When drugs are combined, clinically important drug–drug interactions may occur and lead to unexpected changes in plasma or intracellular drug concentrations. This may consequently reduce antiviral efficacy or increase drug toxicity [2]. Few interactions between NRTIs and other antiretrovirals have been described. All NRTls are inactive prodrugs and must enter cells to be phosphorylated by cellular kinases to triphosphate analogues before exerting their antiretroviral activity [3,4]. Although the plasma pharmacokinetics of NRTls can be easily examined, they show no clear correlations with safety or efficacy, whereas the concentrations of the intracellular triphosphates are the critical parameter to predict antiviral efficacy and toxicity in vivo [5].
Abacavir (ABC) is an NRTI that is commonly used in antiretroviral regimens. It is a synthetic guanosine analogue, converted inside cells to carbovir 5′-triphosphate (CBV-TP), an active metabolite that is a potent inhibitor of HIV reverse transcriptase [6]. ABC has demonstrated antiretroviral efficacy when dosed either 300 mg twice daily or 600 mg once daily [7,8]. The intracellular half-life of CBV-TP has been estimated to range between 12 and 20 h, with exposures varying between men and women [9]. However, in the initial treatment of HN-infected patients with screening HIV RNA higher than 100,000 copies/ml, ABC and lamivudine was shown to be less effective when compared with the combination of tenofovir disoproxil fumarate and emtricitabinc with either efavirenz or atazanavirlritonavir (ACTG 5202) [10].
The primary routes of elimination of ABC are metabolism by alcohol dehydrogenase and glucuronyl transferase. ABC is not meta bolized by cytochrome P450 (CYP450), neither is it an inducer or an inhibitor of this system. High doses of ethanol have been shown to increase ABC plasma exposure by 41% and to lengthen its plasma elimination half-life [6]. In combination with boosted protease inhibitors, ABC plasma exposure is decreased (40% with tipranavir, 32% with lopinavir and 17% with atazanavir) due to induction of glucuronidation caused by ritonavir coadministration [11,12]. However, the clinical significance of these interactions has not been established, as no data on the intracellular concentrations of CBV-TP were available. It is unknown whether the decrease in ABC plasma concentration leads to a change in intracellular concentrations of the active metabolite.
The protease inhibitor darunavir is approved for the treatment of HIV in combination with low-dose ritonavir [13]. As previously shown for other protease inhibitors, coadministration with ABC may cause a decrease in its plasma exposure with unknown consequences on the intracellular concentrations of CBV-TP. However, data on this combination are not currently available.
Raltegravir targets the HIV integrase enzyme. It is predominantly eliminated by glucuronidation mediated by uridinc 5′-diphospho (UDP)-glucuronosyltransferases (UGT) 1Al. Raltegravir has a low potential to be involved in drug–drug interactions as it is not a substrate, an inhibitor or an inducer of CYP450 isoenzymes [14].
As changes in ABC concentrations are expected with ritonavir-boosted darunavir but not with raltegravir, this study aims to investigate the plasma pharmacokinetics of ABC and the intracellular concentrations of CBV-TP in the absence and in the presence of darunavir/ritonavir or raltegravir in HIV-infected subjects.
Methods
Subjects
Adult male and non-pregnant, non-lactating female subjects, with confirmed HIV-1 antibody-positive status, were recruited. In order to determine eligibility, subjects were screened to meet the following criteria: age between 18 and 65 years, receiving on-going treatment with ABC 600 mg once daily plus two NRTIs (excluding tenofovir), and CD4+ T-cell count >100 cells/mm3.
Subjects were excluded based on the presence of any active clinically significant disease or AIDS-defining illness, body mass index (BMI)>30 kg/m2, evidence of uncontrolled HIV replication (viral load >400 copies/ml) and intake of disallowed concomitant therapies.
Approval for the study was obtained from the Brighton Research Ethics Committee (UK) and written informed consent was obtained from each study subject before study procedures were conducted.
Study design
This was an open-label prospective randomized two-phase, cross-over pharmacokinetic study conducted at the Clinical Trial Unit of the St Stephen’s AIDS Trust, Chelsea and Westminster Hospital (London, UK).
The study was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki, and applicable regulatory requirements (EudraCT, 2007-003540-29; clinicaltrials.gov identifier, NCT00765271).
Assuming a wide intra-individual variability in intracellular CBV-TP concentrations (40%), a sample size of 18 subjects completing the study would allow us to detect a significant difference in drug concentration with 80% power during the different study phases using a two-tailed test at α=0.05.
At screening, subjects were clinically assessed and routine laboratory investigations were performed. The safety and tolerability of study medications were evaluated throughout the study using the AIDS Clinical Trials Group (ACTG) Grading Scale.
Following successful screening, eligible subjects on ABC (600 mg once daily) plus two NRTIs (excluding tenofovir) underwent a 24 h pharmacokinetic assessment on day 1 for the determination of plasma ABC and intracellular CBV-TP concentrations.
On day 2, subjects were randomly assigned (1:1) to either darunavir/ritonavir 900/100 mg once daily or raltegravir 400 mg twice daily for 14 days. A second 24 h pharmacokinetic assessment was performed on day 15, followed by a switch to the alternative additional therapy, raltegravir 400 mg twice daily or darunavir/ritonavir 900/100 mg once daily from day 16 for 14 days with a final 24 h pharmacokinetic assessment on day 29.
At the time of the study, darunavir/ritonavir 600/1 00 mg twice daily was approved for treatment of HIV infection but 800/100 mg once daily was not. It was predicted that with emerging data on the effectiveness of once daily darunavir/ritonavir, it would eventually be more commonly prescribed when possible. Therefore, a dose of darunavir/ritonavir of 900/100 mg once daily was chosen as at the time (2008) the 300 mg and not the 400 mg tablets were commercially available.
On pharmacokinetic assessment days 1, 15 and 29 following a standardized breakfast, all subjects had serial blood samples drawn pre-dose at 0.5, 2, 3, 4, 6, 8, 10,12 and 24 h post-dose for determinations of plasma ABC and intracellular CBV-TP. Blood samples were collected into CPT tubes (BD Vacutainer, Franklin Lakes, NJ, USA), as previously described [9]. An additional blood sample was collected for plasma ABC 1 h after dosing. Darunavir, ritonavir and rahegravir plasma concentrations were measured on days 15 and 29 over the 24 and 12 h dosing intervals, respectively.
Efficacy and safety assessments
Efficacy, safety and tolerability of the study medications were assessed by questionnaires, physical examination and laboratory parameters performed at regular intervals during the study.
Analytical and pharmacokinetic methods
Plasma samples were analysed for ABC concentrations by GlaxoSmithKline (Research Triangle Park, NC, USA) and for darunavir, ritonavir and raltegravir by the Department of Pharmacology at the University of Liverpool (Liverpool, UK) using previously described validated methods [9,15].
Peripheral blood mononuclear cell (PBMC) extracts were analysed for CBV-TP by Taylor Technology (Princeton, NJ, USA) using a validated analytical method, as reported by Moyle et al. [9].
Pharmacokinetic and statistical analysis
Plasma darunavir, ritonavir, raltegravir and ABC and intracellular CBV-TP maximum concentrations (Cmax) and trough concentrations (Ctrough) were derived. Area under the curve (AUC) values from 0 to 12 h (for raltegravir) and 0 to 24 h for darunavir, ritonavir, plasma ABC and intracellular CBV-TP were calculated using Phoenix™ WinNonlin® (version 1.1; Mountain View, CA, USA), by non-compartmental linear–linear trapezoidal method. Inter-individual variability in concentrations was assessed by calculating the coefficient of variation (CV = standard deviation/mean×100).
Intracellular CBV-TP concentrations (C) in fmol/million cells, were calculated based on the CBV-TP concentration in PBMC extracts reported in ng/ml and cell counts (PBMCCT) using the formula C(fmol/million cells) = C(ng/ml)×106/(molecular mass×PBMCCT/1 ml), where molecular mass is that of CBV-TP (487.3 Da).
Within subject changes of drug concentrations (drug alone versus drug combination) were assessed by calculating geometric means (GMs), GM ratios (GMRs) and 90% CI. The CI were first determined llsing logarithms of the individual GMR values and then expressed as linear values. The changes in pharmacokinetic parameters were considered significant when the CI for the GMR did not cross the value of one. Simple linear regression analysis was used to assess the relationship between plasma ABC and intracellular CBV-TP.
Results
Demographic and clinical characteristics
A total of 22 subjects were enrol1ed into the study of whom 20 were dosed and 19 (2 females) completed. Two subjects withdrew consent and one was not eligible as he had a positive urine illicit drug test on day 1 prior to dosing. The mean (±SD) age of the 19 subjects who completed the study was 45 (±8) years. Most subjects were Caucasian (n =15), four were African and one was Asian. All subjects had undetectable plasma HIV RNA at screening and their median (range) CD4+ T-cell count was 582 (227–1,129) cel1s/mm3. Concurrent antiretroviral medications administered with the study medications included zidovudine and lamivudine (on the pharmacokinetic assessment days comedications were administered at the same time as ABC, following a standardized breakfast).
Study medications were generally well-tolerated and no grade 3 or 4 adverse events or changes in laboratory parameters were reported. Thirteen individuals experienced at least one adverse event, six were considered mild (grade 1) and seven moderate (grade 2). The most common adverse event reported was headache.
All subjects maintained an undetectable viral load throughout the study.
Pharmacokinetics
Pharmacokinetic results and comparison of steady-state plasma ABC and intracellular CBV-TP pharmacokinetic parameters in the absence and in the presence of darunavir/ritonavir or raltegravir are summarized in Tables 1 and 2, respectively.
Table 1.
Steady-state plasma ABC pharmacokinetic parameter when administered without and with darunavir/ritonavir or raltegravira
| Geometric mean (90% CI) |
|||
|---|---|---|---|
| Plasma | Cmax, ng/ml | Ctrough, ng/ml | AUC0–24, ng•h/ml |
| ABC | 4,009 (3,690, 4,668) | 4.3 (3.8, 7.8) | 13,536 (12,175, 16,375) |
| CV,% | 29 | 87 | 37 |
| ABC + darunavir/ritonavir | 3,115 (2,883, 3,673) | 2.7 (2.4, 5.0) | 9,882 (8,992, 11,882) |
| CV, % | 30 | 88 | 35 |
| GMR (90% CI) | 0.78 (0.69, 0.87) | 0.62 (0.50, 0.77) | 0.73 (0.66, 0.80) |
| ABC + raltegravir | 4,261 (3,934, 4,870) | 3.6 (2.9, 10.5) | 13,969 (12,824, 16,111) |
| GMR (90% CI) | 1.06 (0.95, 1.18) | 0.83 (0.62, 1.11) | 1.03 (0.97, 1.10) |
| CV, % | 27 | 142 | 29 |
n=19. ABC, abacavir; AUC0–24, area under the curve 0–24 h; Cmax, maximum concentration; Ctrough, trough concentration; CV, coefficient of variation = (standard deviation/mean)×100; GMR, geometric mean ratio.
Table 2.
Steady-state intracellular CBV-TP pharmacokinetic parameters when administered without and with darunavir/ritonavir or raltegravira
| Geometric mean (90% CI) |
|||
|---|---|---|---|
| Intracellular | Cmax, fmol/million cells | Ctrough, fmol/million cells | AUC0–24, fmol•h/million cells |
| CBV-TP | 329 (298, 450) | 107 (96, 143) | 4,459 (4,060, 5,802) |
| CV,% | 53 | 47 | 43 |
| CBV-TP + darunavir/ritonavir | 331 (274, 523) | 76 (71, 144) | 3,864 (3,306, 5,111) |
| CV,% | 78 | 75 | 47 |
| GMR (90% CI) | 0.98 (0.79, 1.23) | 0.68 (0.48, 0.95) | 0.88 (0.72, 1.07) |
| CBV-TP + raltegravir | 362 (313, 627) | 68 (68, 138) | 4,347 (3,935, 5,872) |
| CV,% | 84 | 81 | 47 |
| GMR (90% CI) | 1.07 (0.85, 1.35) | 0.57 (0.33, 1.00) | 0.96 (0.76, 1.20) |
n=19. ABC, abacavir; AUC0–24, area under the curve 0–24 h; CBV-TP, carbovir triphosphate; Cmax maximum concentration; Ctrough, trough concentration; CV, coefficient of variation = (standard deviation/mean)×100; GMR, geometric mean ratio.
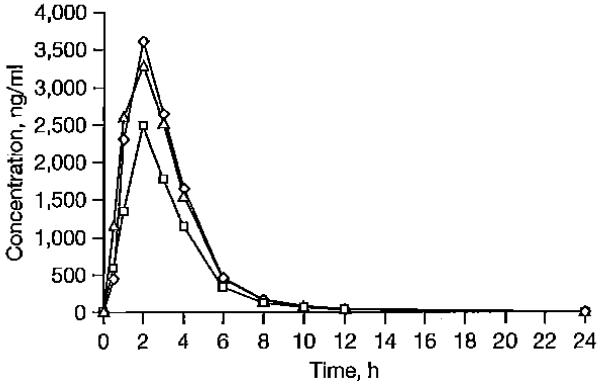
Plasma ABC profiles are illustrated in Figure 1. ABC AUC, Ctrough and Cmax were decreased by 27%, 38% and 22 %, respectively, in the presence of darunavir/ritonavir. No significant changes in ABC concentrations were observed in the presence of raltegravir.
Figure 1.
Plasma concentrations of ABC
Concenlration-time protiles or geomelric mean plasma abacavir (ABC) in the absence of darunavir/ritonavir and raltegravir (diamonds), during coadministration with darunavir/ritonavir (squares), and during co-administration with raltegravir (triangles).
Inter-individual variability in ABC Ctrough was wide (>87%), especially in the presence of raltegravir (Table 1).
GM darunavir and ritonavir plasma AUC0–24 were 84,303 and 5,209 ng•h/ml, respectively. GM raltegravir plasma AUC0–12 was 2,412 ng•h/ml. These were similar to those previously measured in HIV-infected individuals (historical controls) [16,17].
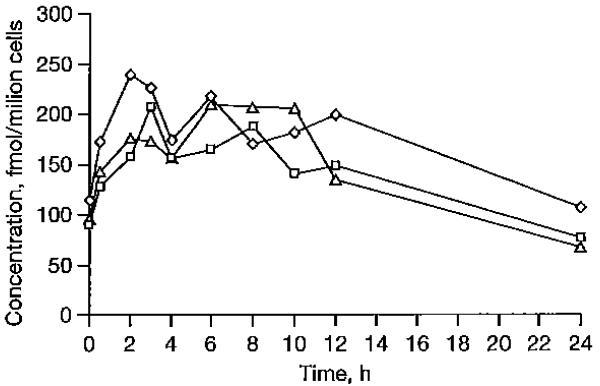
Intracellular CBV-TP concentration profiles without and with darunavir/ritonavir and raltegravir are illustrated in Figure 2. Intracellular CBV-TP Ctrough was lower (32%) in the presence of darunavir/ritonavir, while no other significant change was measured for the other pharmacokinetic parameters with darunavir/ritonavir. Raltegravir coadministration did not lead to any significant alteration in CBV-TP concentrations, with the exception of a 43% decrease in CBV-TP Ctrough However, the latter did not seem to be statistically significant, as the 90% CI of the GMR ranged between 0.33 and 1.00.
Figure 2.
Intracellular concentrations of CBV-TP
Concentration-time profiles of geometric mean intracellular carbovirtriphosphate (CBV-IP) in the absencc of darunavir/ritonavir and raltegravir (diamonds), during coadministration of abacavir and darunavir/ritonavir (squares). and during coadministration of abacavir and raltegravir (triangles).
During coadministration of ABC and darunavir/ritonavir or raltegravir, the inter-individual variability in intracellular CBV-TP pharmacokinetic parameters was wide (>43 %), especially for CBV-TP Ctrough in the presence of raltegravir (CV of 81 %).
We did not observe a sequence effect, as intracellular CBV-TP pharmacokinetic parameters were not different when in the presence of darunavir/ritonavir or raltegravir irrespective of the order in which the drugs were given (that is, ABC then ABC plus raltegravir then abacavir plus darunavir/ritonavir versus ABC then ABC plus darunavir/ritonavir then ABC plus raltegravir).
As shown by previous studies [17], we observed a weak correlation between plasma ABC and intracellular CBV-TP concentrations for ABC alone (r2=0.013, P=0.65), and in the presence of darunavir/ritonavir (r2=0.108, P=0.23) or raltegravir (r2=0.339, P=0.01).
Discussion
We report here the steady-state pharmacokinetics of plasma ABC and intracellular CBV-TP following the administration of ABC 600 mg once daily to HIV-infected individuals, in the absence and in the presence of darunavir/ritonavir (900/100 mg once daily) or raltegravir (400 mg twice daily).
Although ABC plasma pharmacokinetic parameters were decreased in the presence of darunavir/ritonavir, only CBV-TP intracellular Ctrough was significantly decreased (32 %). No significant changes were observed for either ABC plasma or CBV-TP intracellular concentrations in the presence of raltegravir; however, a wider inter-individual variability in both ABC and CBV-TP Ctrough was observed during coadministration.
The combinations of ABC and darunavir/ritonavir or raltegravir were well-toleratcd, with mild or moderate adverse events limited to a small number of subjects.
The use of darunavir/ritonavir and raltegravir in combination with NRTIs is increasing. Whilst darunavir and ritonavir are substrates and inhibitors of CYP450, raltegravir is metabolized by Phase II reactions, mainly glucuronidation [14,16,18].
NRTIs are generally not metabolized by hepatic CYP450 enzymes and their metabolism depends on intracellular enzymes such as nucleoside kinases, 5′-nucleotidases, purine and pyrimidine nucleoside monophosphate kinases [3].
Although ABC plasma concentrations have been shown to be decreased by the coadministration of ritonavir-boosted protease inhibitors [19], no data were available on the concentrations of CBV-TP. In our study, only the CBV-TP Ctrough was affected by a decrease in the plasma concentrations of ABC seen in the darunavir/ritonavir arm. Cmax and AUC0–24 were not significantly different.
Interpretation of data showing a decrease in intracellular TP concentrations is complex because the antiviral activity of NRTIs is not only dependent on the levels of the TP formed but also on the endogenous concentrations of the dNTPs that the NRTI-TP competes with for incorporation into the proviral DNA [20]. Therefore, the clinical significance of our results (especially in HIV-infected individuals harbouring resistant HIV virus) remains unclear. However, this is the first study showing a change in intracellular TP concentrations, as a result of a drug interaction involving an NRTI and an antiretroviral belonging to a different class. Intracellular drug interactions were only previously described between different NRTIs competing for the enzymes responsible for their activation [21].
A further matter complicating the interpretation of these data is the lack of target concentrations of CBV-TP: intracellular measurements of NRTI-TP are highly variable [22] and the specific target ranges associated with drug efficacy have not been established.
Data from ACTG 5202 indicated that the lower efficacy of the ABC-based regimen at high viral loads was observed in male subjects but not in females [10]. A gender difference in intracellular phosphorylation of ABC was previously described, with women showing higher intracellular CBV-TP exposure than men [9]. Our data potentially support the hypothesis of an interaction between ABC and atazanavir/ritonavir or efavirenz leading to lower CBV-TP concentrations may have contributed to the lower efficacy of ABC-based regimens and that this was most evident in males who tend to have lower CBV-TP concentrations regardless of the partner agents. The lack of a significant interaction between ABC and raltegravir suggests that the efficacy of this regimen should not be affected. A single arm study of ABC with lamivudine and raltegravir has demonstrated good efficacy in the presence of high viral loads [23].
Due to the complexity of sampling procedures and the intrinsic variability in ABC intracellular uptake and TP formation, we observed a large inter-individual variability in CBV-TP intracellular concentrations, especially in the presence of darunavir/ritonavir and raltegravir (>70%). The increased variability observed in intracellular CBV-TP following the addition of darunavir/ritonavir and raltegravir may be due to variability in drug intracellular uptake or intracellular activation of the prodrug. The varia bility in plasma ABC was limited with the exception of the low concentrations measured at the end of the dosing interval.
In conclusion, this study shows that interactions between protease inhibitors and NRTIs are not only responsible for changes in NRTI plasma concentrations, but also alterations in the intracellular active anabolite concentrations may occur. However, a better understanding of the clinical consequences of these changes is needed.
Acknowledgements
The authors would like to thank GlaxoSmithKline, UK and the St Stephen’s AIDS Trust for funding the study and Merck for proving raltegravir. Some of the results of this study were presented at the 12th European AIDS Conference (11–14 November 2009, Cologne, Germany; abstract PE4.3/2).
Footnotes
Disclosure statement
AJ, DB, SK, GM, BG and MB have received travel and research grants from and have been advisers for Tibotec, Roche, Pfizel; GlaxoSmithKline, Bristol–Myers Squibb, Merck, Abbott and Boehringer–Ingelheim. All other authors declare no competing interests.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; [Accessed 5 September 2011]. 2009. Updated 10 January 2011. Available from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.Boffito M, Acosta E, Burger D, et al. Therapeutic drug monitoring and drug-drug interactions involving antiretroviral drugs. Antivir Ther. 2005;10:469–477. [PubMed] [Google Scholar]
- 3.Piliero PJ. Pharmacokinetic properties of nucleoside/nucleotide reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2004;37(Suppl):S2–S12. doi: 10.1097/01.qai.0000137001.40505.56. [DOI] [PubMed] [Google Scholar]
- 4.Kewn S, Hoggard PG, Sales SD, et al. Development of enzymatic assays for quantification of intracellular lamivudinc and carbovir triphosphate levels in peripheral blood mononuclear cells from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2002;46:135–143. doi: 10.1128/AAC.46.1.135-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17:2159–2168. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 6.Foster RH, Faulds D. Abacavir. Drugs. 1998;55:729–736. doi: 10.2165/00003495-199855050-00018. [DOI] [PubMed] [Google Scholar]
- 7.Moyle GJ, DeJesus E, Cahn P, et al. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J Acquir Immune Defic Syndr. 2005;38:417–425. doi: 10.1097/01.qai.0000147521.34369.c9. [DOI] [PubMed] [Google Scholar]
- 8.Smith KY, Patel P, Fine D, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS. 2009;23:1547–1556. doi: 10.1097/QAD.0b013e32832cbcc2. [DOI] [PubMed] [Google Scholar]
- 9.Moyle G, Boffito M, Fletcher C, et al. Steady-state pharmacokinetics ot abacavir in plasma and intracellular carbovir triphosphate following administration of abacavir at 600 milligrams once daily and 300 milligrams twice daily in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2009;53:1532–1538. doi: 10.1128/AAC.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sax PE, Tierney C, Collier AC, et al. Abacavir-Iamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aptivus (tipranavir) Package insert. Boehringer–Ingelheim; Ridgefield, CT, USA: 2011. [Google Scholar]
- 12.Waters L, Fisher M, Winston A, et al. A Phase IV, double-blind, multicentre, randomized, placebo-controlled, pilot study to assess the feasibility of switching individuals receiving efavirenz with continuing central nervous system adverse events to etravirine. AIDS. 2011;25:65–71. doi: 10.1097/QAD.0b013e328341685b. [DOI] [PubMed] [Google Scholar]
- 13.McKeage K, Perry CM, Kearn SJ. Daruavir: a review of its use in the management of HIV infection in adults. Drugs. 2009;69:477–503. doi: 10.2165/00003495-200969040-00007. [DOI] [PubMed] [Google Scholar]
- 14.Burger DM. Raltegravir: a review of its pharmacokinetics, pharmacology and clinical studies. Expert Opin Drug Metab Toxicol. 2010;6:1151–1160. doi: 10.1517/17425255.2010.513383. [DOI] [PubMed] [Google Scholar]
- 15.Else L, Watson V, Tjia J, et al. Validation of a rapid and sensitive high-performance liquid chromatographytandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1455–1465. doi: 10.1016/j.jchromb.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Curran A, Gutirerrez M, Deig E, et al. Efficacy, safety and pharmacokinetics of 900/100 mg of darunavir/ritonavir once daily in treatment-experienced patients. J Antimicrob Chemother. 2010;65:2195–2203. doi: 10.1093/jac/dkq295. [DOI] [PubMed] [Google Scholar]
- 17.Hoggard PG, Sales SD, Kewn S, et al. Correlation between intracellular pharmacological activation of nucleoside analogues and HIV suppression in vitro. Antivir Chem Chemother. 2000;11:353–358. doi: 10.1177/095632020001100601. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz M, Morales-Ramirez JO, Nguyen BY, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43:509–515. doi: 10.1097/QAI.0b013e31802b4956. [DOI] [PubMed] [Google Scholar]
- 19.Waters LJ, Moyle G, Bonora S, et al. Abacavir plasma pharmacokinetics in the absence and presence of atazanavir/ritonavir or lopinavir/ritonavir and vice versa in HIV-infected patients. Antivir Ther. 2007;12:825–830. [PubMed] [Google Scholar]
- 20.Gao WY, Shirasaka T, Johns DG, Broder S, Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Invest. 1993;91:2326–2333. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray AS. Intracellular interactions between nucleos(t)ide inhibitors of HIV reverse transcriptase. AIDS Rev. 2005;7:113–125. [PubMed] [Google Scholar]
- 22.Goicoechea M, Jain S, Bi L, et al. Interlaboratory measurement differences in intracellular carbovir triphosphate concentrations in HIV-infected patients: sources of variability in processing, shipping, and quantitation. J Clin Pharmacol. 2010;50:968–974. doi: 10.1177/0091270009352186. [DOI] [PubMed] [Google Scholar]
- 23.Young B, Vanig T, Dejesus E, et al. A pilot study of abacavir/lamivudine and raltegravir in antiretroviral-naive HIV-1-infected patients: 48-week results of the SHIELD Trial. HIV Clin Trials. 2010;11:260–269. doi: 10.1310/hct1105-260. [DOI] [PubMed] [Google Scholar]