Figure 1.
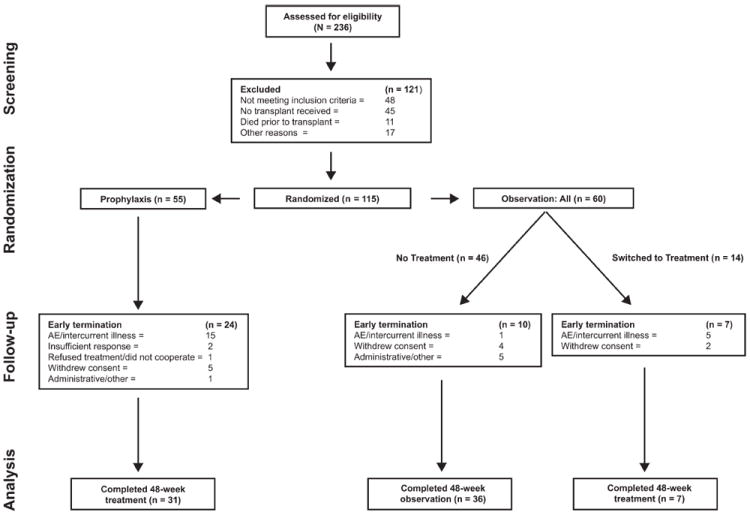
Overview of the study and its subjects. Patients were randomized 10 to 26 weeks post-LT to receive peginterferon alfa-2a/ribavirin therapy either prophylactically or upon observation of HCV recurrence. The study duration was 120 weeks: prophylaxis patients received 48 weeks of treatment and 72 weeks of follow-up, observation patients who received no treatment received 48 weeks of observation and 72 weeks of follow-up, and observation patients who switched to treatment received 0 to 48 weeks of observation followed by 48 weeks of treatment and 24 to 72 weeks of follow-up.
